Oral preparation capable of dosing fertility-controlling drugs by positioning at small intestine and production method thereof
A technology for contraceptives and oral preparations, applied in pharmaceutical formulations, drug combinations, drug delivery, etc., can solve problems such as inaccurate drug dosage, drug overdose, and increase in adverse reactions, and achieve the effect of ensuring the effectiveness of drugs and ensuring effective doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
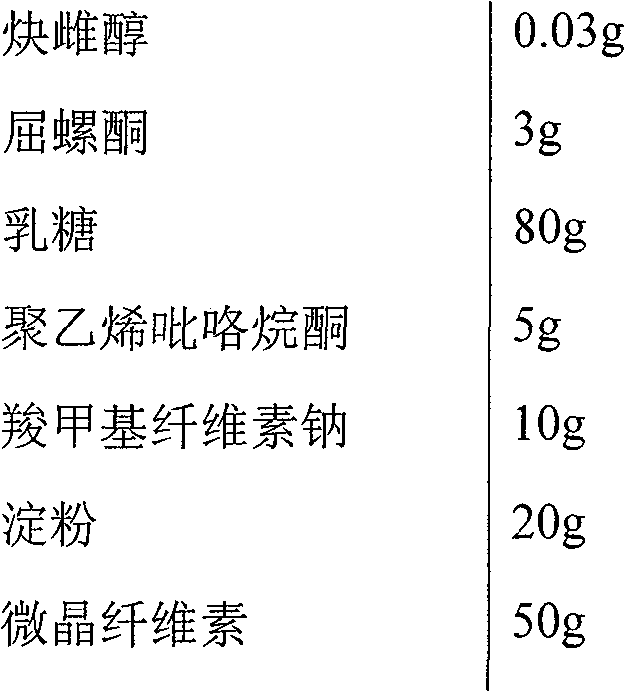
[0033] Drospirenone Ethinylestradiol Enteric-Coated Tablets
[0034]
[0035] Mix the above materials, add water to granulate, dry, granulate, and press into 1000 round tablets with a diameter of 8 mm, each containing 0.03 mg of ethinyl estradiol and 3 mg of drospirenone.
[0036] At the same time, 85% ethanol is used as a solvent, and a 10-15% suspension is prepared with a special coating material containing cellulose acetate phthalate, and after mixing, a layer is coated on the prepared tablet. The coated tablet made was stirred in 750ml of 0.1N hydrochloric acid (PH1) for 2 hours, without any disintegration or dissolution was not more than 10%; in 0.2N sodium phosphate solution (adjusted with 1N HCL / NaOH PH to 6.8; add 5g sodium lauryl sulfate), the prepared coated tablet was stirred therein, and disintegrated completely in about 10 minutes, and the dissolution rates of ethinyl estradiol and drospirenone were respectively greater than 80% by sampling after 1 hour. %.
Embodiment 2
[0038] Mifepristone enteric-coated tablets / capsules
[0039]
[0040] (1) Mix the above materials, add water to granulate, dry, granulate, and press into 1000 tablets, each containing 25 mg of mifepristone.
[0041] At the same time, 80% ethanol is used as a solvent, and a 10-15% suspension is prepared with a special coating material containing cellulose acetate phthalate, and after mixing, a layer is coated on the prepared tablet.
[0042] or
[0043] (2) Mix the above materials, add water to granulate, dry, and pour the whole grain into enteric-coated No. 3 capsules approved by the state. Each capsule contains 25 mg of mifepristone.
[0044] (3) The coated tablet or capsule made above was stirred in 0.1N hydrochloric acid (PH1) of 750ml for 2 hours, without any disintegration or dissolution being no more than 10%; in 0.2N sodium phosphate solution ( Use 1N HCL / NaOH to adjust the pH to 6.8; add 5g sodium lauryl sulfate), and stir the prepared coated tablet or capsule wit...
Embodiment 3
[0046] Compound Levonorgestrel Enteric-Coated Tablets
[0047]
[0048]
[0049] Mix the above materials, add water to granulate, dry, granulate, and press into 1000 round tablets with a diameter of 8 mm, each containing 0.15 mg of ethinyl estradiol and 0.03 mg of levonorgestrel.
[0050]At the same time, 85% ethanol is used as a solvent, and a 10-15% suspension is prepared with a special coating material containing cellulose acetate phthalate, and after mixing, a layer is coated on the prepared tablet. The coated tablet made was stirred in 750ml of 0.1N hydrochloric acid (PH1) for 2 hours without any disintegration or dissolution was not more than 10%; in 0.2N sodium phosphate solution (adjusted with 1N HCL / NaOH PH to 6.8; add 5g sodium lauryl sulfate), and the prepared coated tablet was stirred therein, and disintegrated completely in about 10 minutes, and after 1 hour, the dissolution rates of ethinyl estradiol and levonorgestrel were sampled and measured respectively...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap