Polypeptide capable of inhibiting excitable and toxic damages and use thereof
An excitatory and peptide technology, applied in applications, peptide sources, animal/human peptides, etc., can solve problems such as motor function damage, toxic side effects, and blocking excitatory synaptic transmission functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
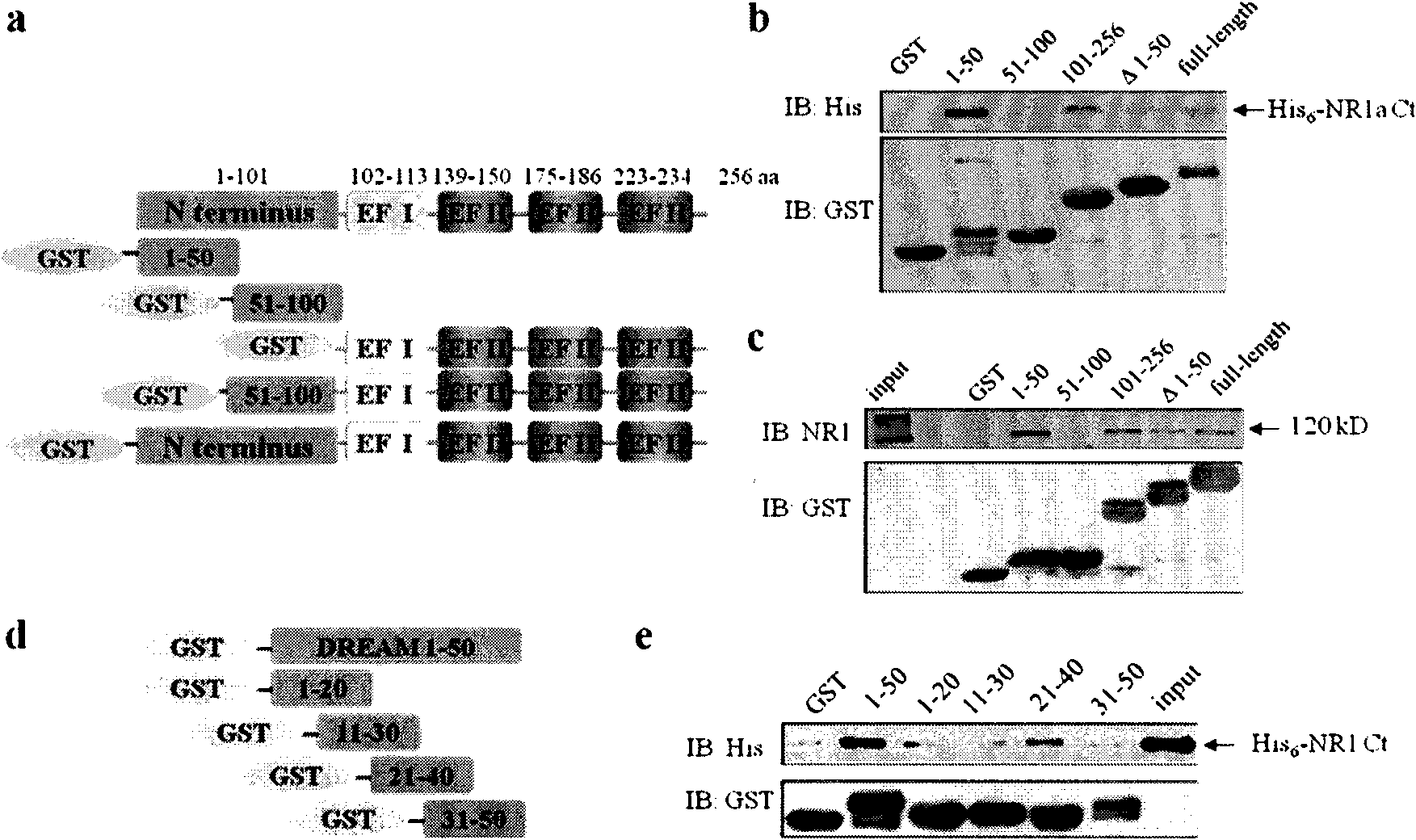
[0028]Example 1, Identification of DREAM protein and NR1 subunit binding site
[0029] The full length of DREAM protein is 256 amino acids, and there is an EF hand domain in each of its 102-113, 139-150, 175-186 and 223-234 amino acids. According to this, the DREAM protein is segmented, the N-terminus is defined as 1-100 positions, the C-terminus is defined as 101-256 positions, contains 4 complete EF hand domains, and the N-terminus is further divided into 1-50 positions and 51-100 positions bit two paragraphs.
[0030] The amino acid sequence of the DREAM protein is shown in SEQ ID NO.1.
[0031] The following peptides were used in Experiment 1: DREAM 1-50 (1-50th amino acid peptide from the N-terminal of the DREAM protein shown in SEQ ID NO.1), DREAM 51-100 (the N-terminus of the DREAM protein shown in SEQ ID NO.1 51-100th amino acid peptide from the end), DREAM 101-256 (the 101-256th amino acid peptide from the N-terminal of the DREAM protein shown in SEQ ID NO.1), DREAM...
Embodiment 2
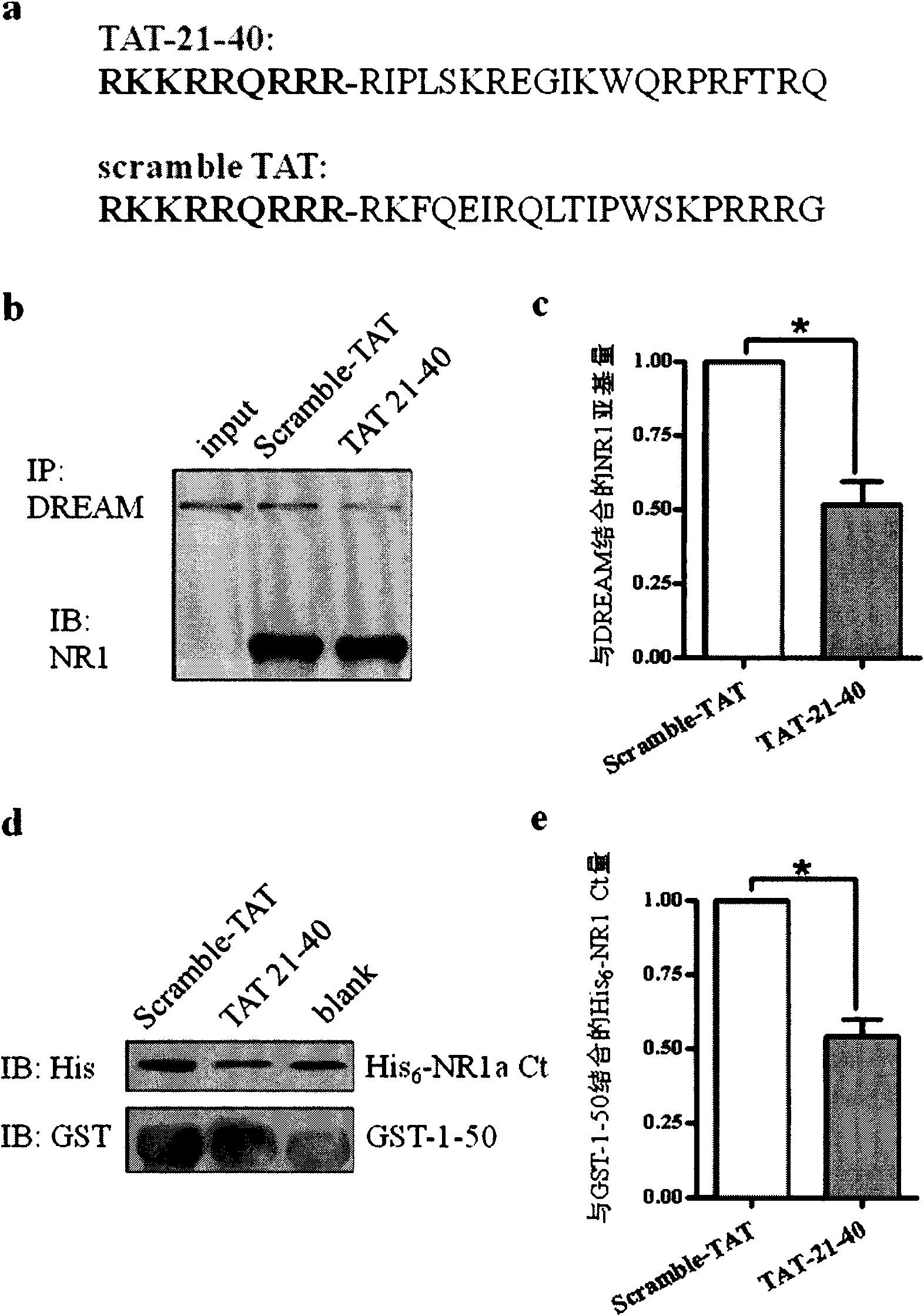
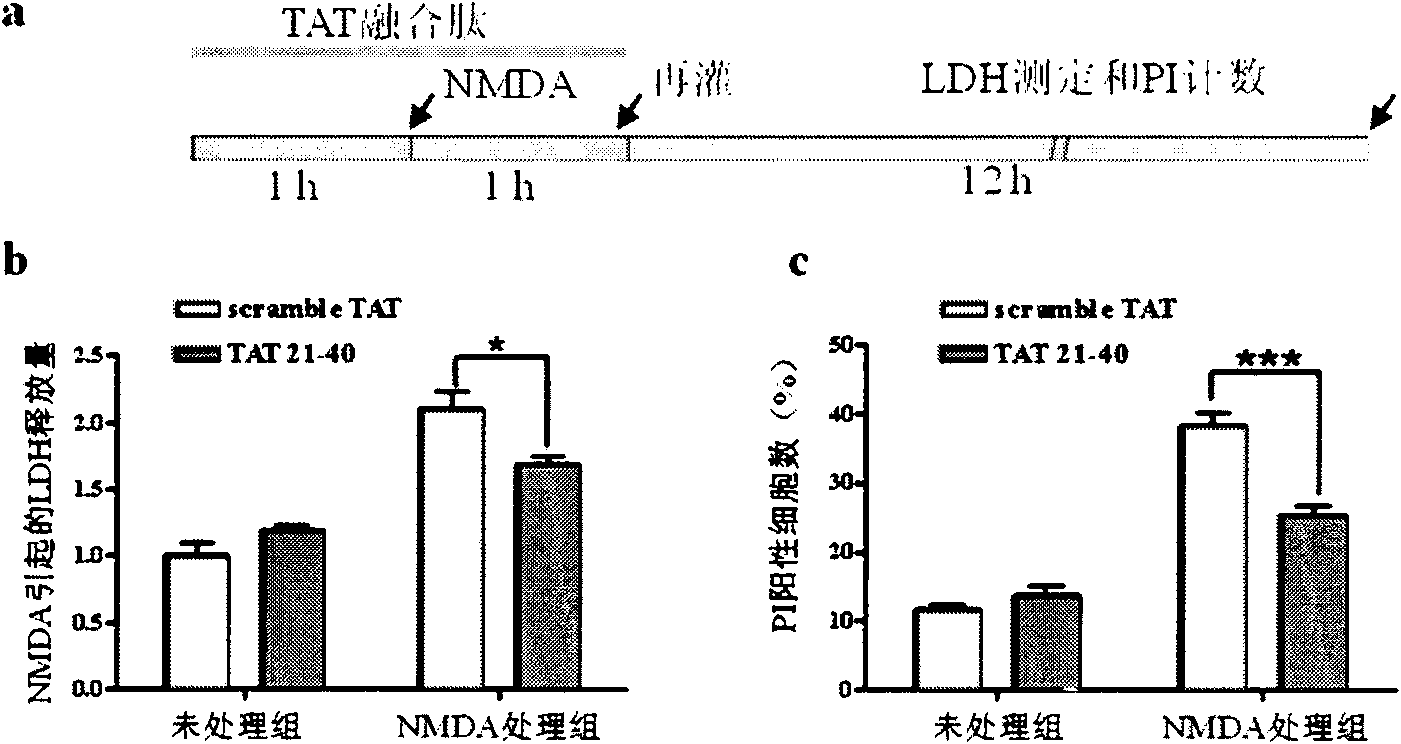
[0070] Example 2, Application of TAT-21-40 in inhibiting excitotoxic injury
[0071] TAT (transcriptional activator protein) penetrating peptide is one of the transmembrane delivery carriers, which can introduce covalently linked polypeptides, proteins, DNA and other molecules into cells across the membrane, and even pass through the blood-brain barrier. Without damage, it has been widely used in the fields of cell biology, gene therapy and pharmacy. According to the main site where the N-terminal of DREAM binds to the NR1 subunit, that is, amino acids 21-40 at the N-terminus, a fusion peptide with membrane-penetrating ability——TAT-21-40 was constructed. The amino acid sequence of the target sequence was scrambled, and scramble-TAT was constructed as a control.
[0072] The amino acid sequence of TAT-21-40 is shown in SEQ ID NO.4, which consists of the TAT transmembrane sequence (RKKRRQRRR) and amino acids 21-40 from the N-terminus of DREAM. TAT-21-40 was synthesized by Jill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com