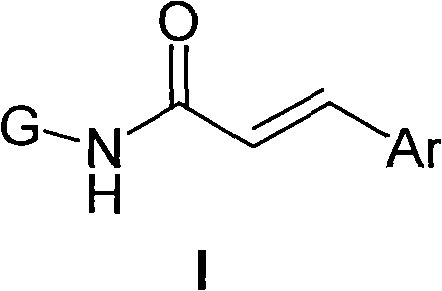
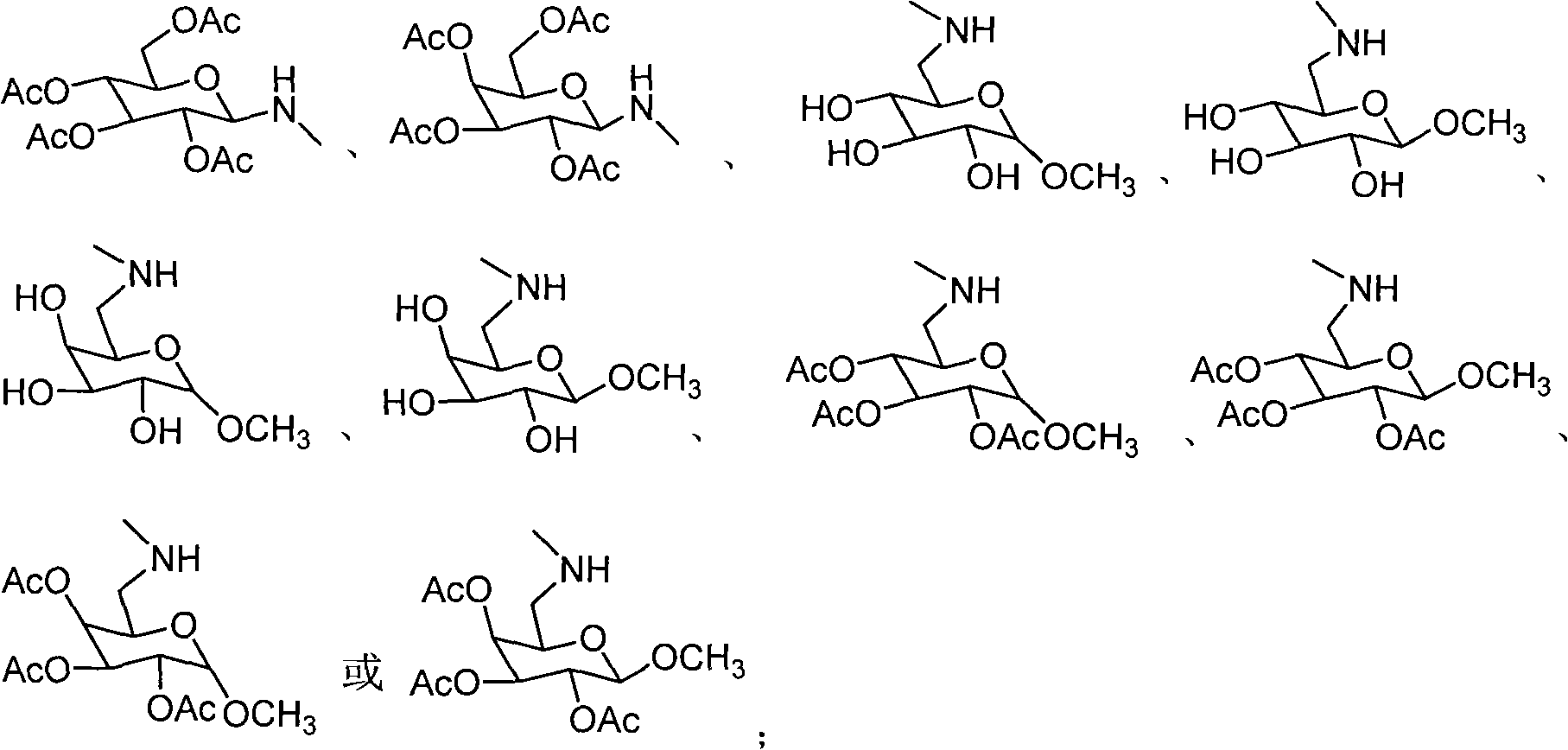
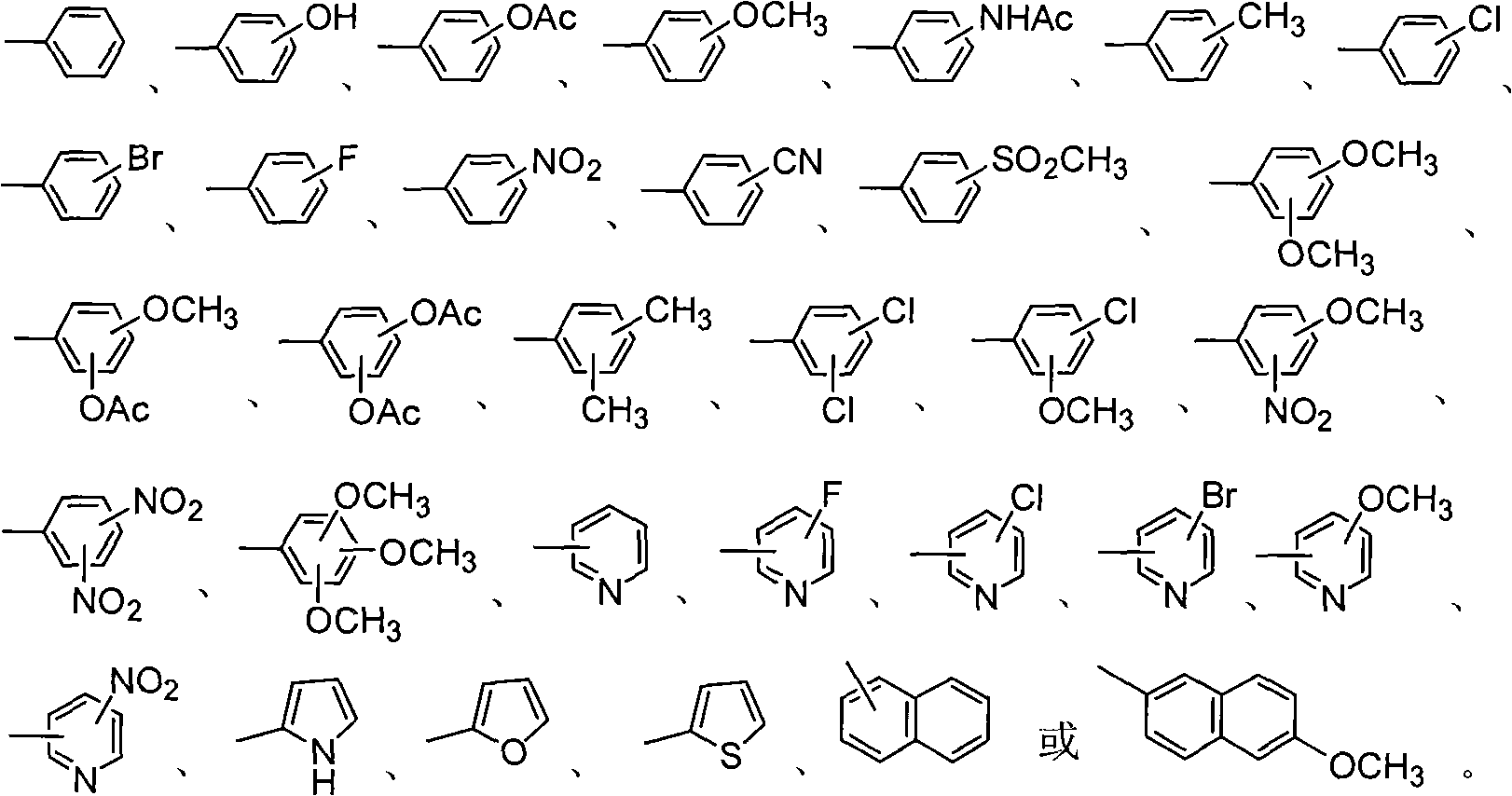
Amino sugar derivative, preparation method thereof and medicinal application thereof
A drug and pharmaceutical technology, applied in sugar derivatives, sugar derivatives, pharmaceutical formulations, etc., can solve the problems of inability to completely eliminate tumor cells, high patient treatment costs, and long drug cycles.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Preparation of N-(2,3,4,6-tetra-O-acetyl-1-deoxy-β-D-glucopyranosyl)-3-phenyl-acrylamide hemihydrate (I-1)
[0118] 1,2,3,4,6-penta-O-acetyl-α-D-glucopyranose (II-1)
[0119] Add ZnCl to the three-neck flask 2 (2.0g) and anhydrous acetic anhydride (54.0g, 50.0mL, 0.52mol), after heating for 30min, zinc chloride was dissolved, and D-glucose powder (10.0g, 0.056mol) was added in batches, and stirred thoroughly. After adding, continue heating on the oil bath (100°C) for about 2h. After cooling, the mixture was poured into 200 mL of ice water and stirred well to decompose unreacted acetic anhydride. An oily substance started to form, and then solidified into a large amount of white precipitate. Stirring was continued for 1 h, filtered, washed with cold water repeatedly, and then dried under an infrared lamp to obtain 18.11 g of a white solid (crude yield 83.58%). The solid was recrystallized from absolute ethanol (70 mL) to obtain a white solid, which was dried under an ...
Embodiment 2
[0140] N-(2,3,4,6-tetra-O-acetyl-1-deoxy-β-D-glucopyranosyl)-3-(3,4-diacetoxyphenyl)-acrylamide semi Preparation of Hydrate (I-5)
[0141] Add 1.5g (8.33mmol) of caffeic acid into a solution formed by dissolving 1.15g (28.75mmol) of NaOH in 11ml of water under cooling in an ice bath, and slowly add Ac 2 O 2.27ml (20.82mmol), the solution became cloudy after the dropwise addition, removed the ice bath and stirred at room temperature for 1.5h. Stop the reaction with 10% H 2 5O 4 Adjust the pH of the solution to about 2-3, continue to stir for 20 minutes, filter, wash the filter cake several times with water, and dry under an infrared lamp to obtain 2.01 g of off-white solid (fully acetylated caffeic acid), with a yield of 91.36%.
[0142] Suspend 0.34 g (1.3 mmol) of fully acetylated caffeic acid in 10 mL of dry CH 2 Cl 2 0.70g (0.47mL, 5.5mmol) of oxalyl chloride was slowly added dropwise, the solution became clear, then a drop of anhydrous DMF was added dropwise, a large ...
Embodiment 3
[0150] N-(2,3,4,6-tetra-O-acetyl-1-deoxy-β-D-glucopyranosyl)-3-(3-methoxy-4-acetoxyphenyl)- Preparation of Acrylamide Monohydrate (I-6)
[0151] Add 1.5g (7.73mmol) of ferulic acid into the solution formed by dissolving 0.8g (20.6mmol) NaOH in 7.7ml of water under cooling in an ice bath, and slowly add Ac 2 O 0.91ml (9.66mmol), the solution became cloudy after the dropwise addition, removed the ice bath and stirred at room temperature for 1.5h. Stop the reaction with 10% H 2 SO 4 Adjust the pH of the solution to 2-3, continue to stir for 20 minutes, filter, wash the filter cake with water several times, and dry it under an infrared lamp to obtain 1.74 g of off-white solid (acetylated ferulic acid), with a yield of 95.35%.
[0152] Suspend 0.31 g (1.3 mmol) of acetylated ferulic acid in 10 mL of dry CH 2 Cl 2 0.70g (0.47mL, 5.5mmol) of oxalyl chloride was slowly added dropwise, the solution became clear, then a drop of anhydrous DMF was added dropwise, a large number of bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com