Benzene sulfonamide hydroxyl derivative and intermediate thereof as well as preparation method and application thereof
A benzenesulfonamide, imine-based technology, applied in the field of new compounds and their preparation, can solve the problem of blanks in molecular imaging research, and achieve good carbonic anhydrase inhibitory activity, less non-specific absorption, and strong hydrophilicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
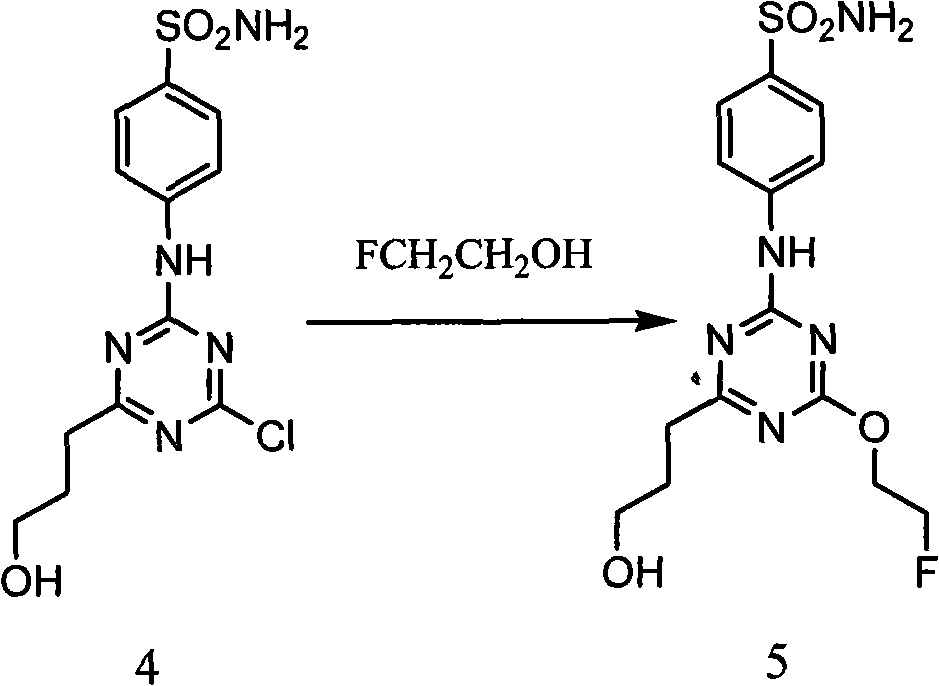
[0055] Example 1 Synthesis of 4-[(4-chloro-6(2--hydroxyethoxy)-1,3,5-triazine-2-imino]benzenesulfonamide (compound 4)
[0056] Dissolve compound 3 (1.6g, 5mmol), NaOH (0.2g, 5mmol) in 8ml of ethylene glycol, stir at a constant temperature of 45°C, follow the reaction with TCL, developer: V (ethyl acetate): V (petroleum ether) = 2: 1, until the reaction of compound 3 is complete, add 10ml of water, cool, the solid precipitates out, filter the solid to obtain a white solid powder, dry in a vacuum oven, separate through a silica gel column (the eluent is pure ethyl acetate, collect Rf= 0.30 component), the solvent was removed by rotary evaporation, and vacuum-dried to obtain 0.95 g of white solid powder with a yield of 55.2%.
[0057] Result identification
[0058] 1 HNMR (DMSO-d6) δ: 10.92 (m, 1H, NH), 7.810-7.757 (d, 4H, aromatic), 7.252 (s, 2H, NH 2 ), 4.940-4-913 (m, 1H, OH), 4.364-4.453 (t, 2H, CH 2 ), 3.709-3.62 (q, 2H, CH 2 ).
[0059] MS m / z (%): 344.0 (M + -H, 100...
Embodiment 2
[0061] Example 2 Synthesis of 4-[(4-chloro-6(2--hydroxyethoxy)-1,3,5-triazine-2-imino]benzenesulfonamide (compound 4)
[0062] Dissolve compound 3 (1.6g, 5mmol), KOH (0.34g, 6mmol) in 13ml of ethylene glycol, stir at a constant temperature of 30°C, follow the reaction with TCL, developer: V (ethyl acetate): V (petroleum ether) = 2: 1, until the reaction of compound 3 is complete, add 10ml of water, cool, the solid precipitates out, filter the solid to obtain a white solid powder, dry in a vacuum oven, separate through a silica gel column (the eluent is pure ethyl acetate, collect Rf= 0.30 component), the solvent was removed by rotary evaporation, and vacuum-dried to obtain 0.62 g of white solid powder with a yield of 36.0%.
Embodiment 3
[0063] Example 3 Synthesis of 4-[(4-chloro-6(2--hydroxyethoxy)-1,3,5-triazine-2-imino]benzenesulfonamide (compound 4)
[0064] Dissolve compound 3 (1.6g, 5mmol), NaOH (0.22g, 5.5mmol) in 11ml of ethylene glycol, stir at a constant temperature of 50°C, follow the reaction with TCL, developing solvent: V (ethyl acetate): V (petroleum ether )=2:1, until compound 3 has reacted, add 10ml of water, cooling, solid precipitation, filter solid, obtain white solid powder, dry in a vacuum oven, separate through silica gel column (eluent is pure ethyl acetate, collect Rf =0.30 component), the solvent was removed by rotary evaporation, and vacuum-dried to obtain 0.85 g of white solid powder with a yield of 49.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com