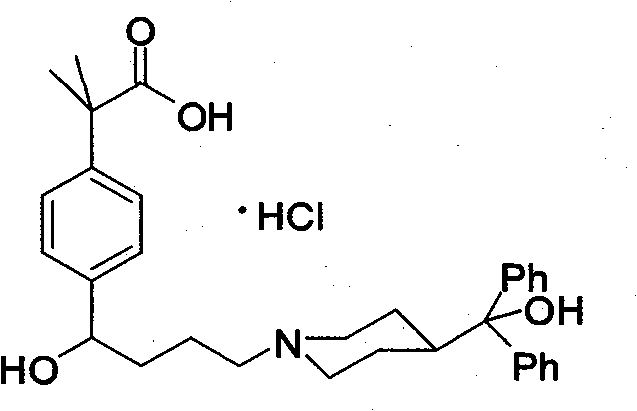
Preparation method of an antiallergic agent fexofenadine hydrochloride
A technology of fexofenadine and hydrochloride, which is applied in the field of preparation of antiallergic drug fexofenadine hydrochloride, can solve the problems of reducing purity and yield, difficult separation of amides, containing amide impurities, etc. The effect of low pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
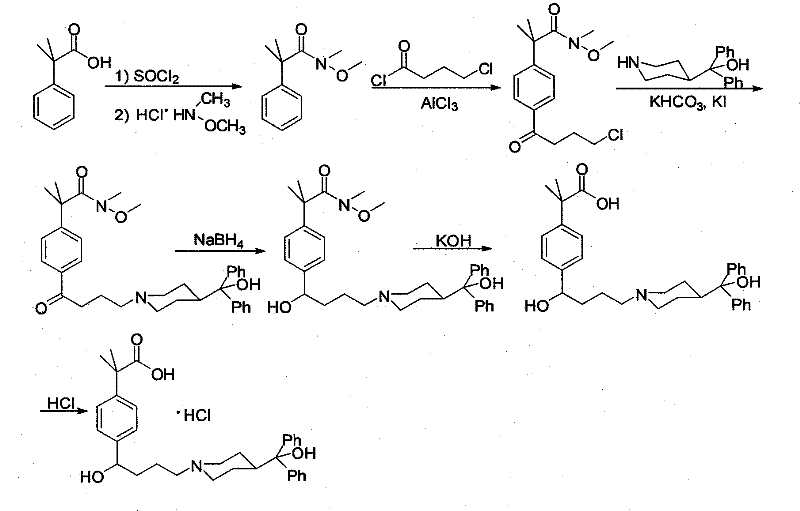
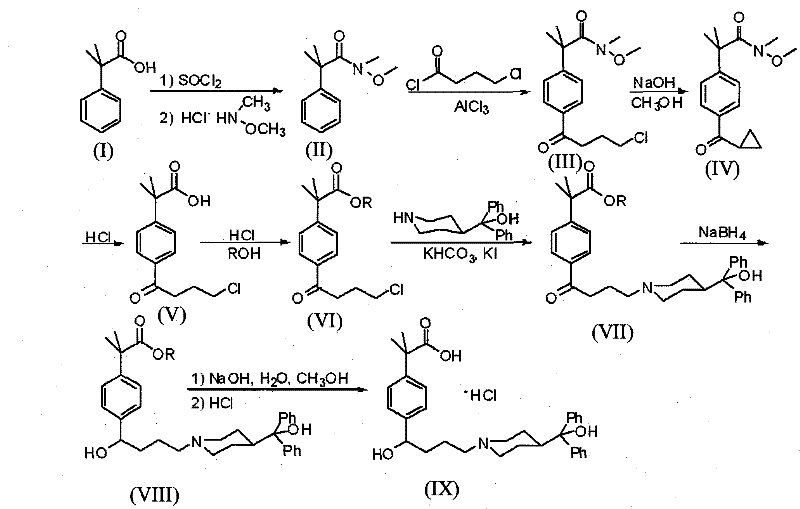
[0041] The present invention starts with N-methyl-N-methoxy-2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanamide (III) as the starting material. N-methyl-N-methoxy-2-[4-(4-chlorobutyryl) phenyl]-2-methylpropionamide is prepared by prior art: (1) 98.4 grams (0.6mol) α , α-dimethylphenylacetic acid was dissolved in 400 milliliters of toluene, 131 milliliters of thionyl chloride was added dropwise at 0°C, stirred at room temperature for 15 hours and then refluxed for 2 hours, and 100 milliliters of toluene and toluene were removed by vacuum distillation after the reaction was completed. Excess thionyl chloride, add 184.6 grams (1.34mol) of potassium carbonate, 58.5 grams (0.6mol) of N, O-dimethylhydroxylamine hydrochloride and 300 milliliters of water, stir and react at room temperature for 4 hours, after the completion of the reaction, the reaction 200 ml of 2N hydrochloric acid was added dropwise to the mixture, and the liquid was separated. The organic phase was washed with 2N hy...
Embodiment 2
[0051] The N-methyl-N-methoxy-2-[4-(4-chlorobutyryl) phenyl]-2-methylpropionamide prepared by the method described in the above-mentioned Example 1 was used as starting material at 250 Add 20.50 grams (82%, 0.30mol) potassium hydroxide and 150 milliliters of anhydrous methanol in the milliliter three-necked flask and stir evenly, then, while stirring, 15.50 grams (0.05mol) N-methyl-N-methoxy-2 -[4-(4-Chlorobutyryl)phenyl]-2-methylpropanamide dissolved in 50 ml of anhydrous methanol to form a uniformly stirred solution was added dropwise to the three-necked flask. The reaction was stirred for 30 hours. After the reaction was completed, anhydrous methanol was distilled off under reduced pressure, 100 ml of water and 100 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. The extracts containing the dichloromethane layer were combined, dried over anhydrous sodium sulfat...
Embodiment 3
[0056] The N-methyl-N-methoxy-2-[4-(4-chlorobutyryl) phenyl]-2-methylpropionamide prepared by the method described in the above-mentioned Example 1 was used as starting material at 250 Add 20.00 grams (0.50mol) of sodium hydroxide and 150 milliliters of dehydrated ethanol in the milliliter three-necked flask, stir well, then, while stirring, 15.50 grams (0.05mol) of N-methyl-N-methoxy-2-[ 4-(4-Chlorobutyryl)phenyl]-2-methylpropanamide was dissolved in 50 ml of absolute ethanol to form a uniformly stirred solution, which was added dropwise to the three-necked flask. After the dropwise addition was completed, the reaction was stirred at 50°C 30 hours. After the reaction was completed, absolute ethanol was distilled off under reduced pressure, 100 ml of water and 100 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. The extracts containing the dichloromethane layer we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com