Organic micromolecular photoelectric material
A technology of optoelectronic materials and small molecules, applied in the field of optoelectronic materials to achieve the effect of good hole transport ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
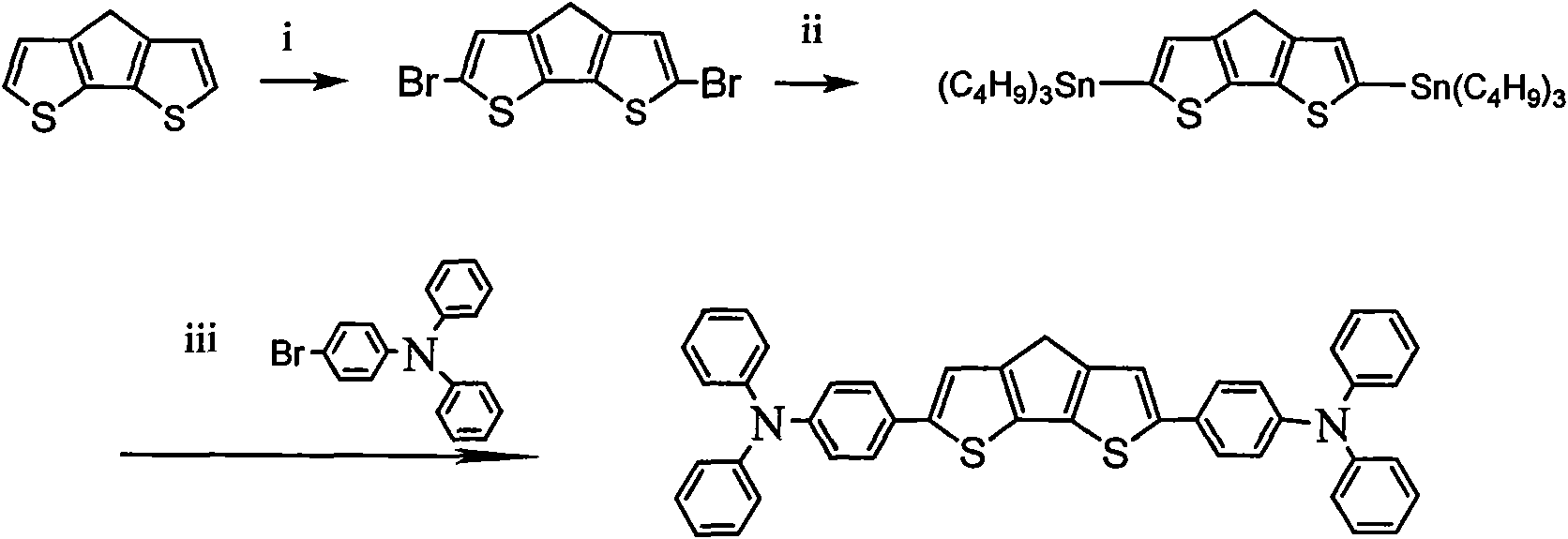
[0044] Embodiment 1: see figure 1 , the preparation of triphenylamine-structure a-triphenylamine:
[0045] Step (1): Add 0.10 mol of cyclopentadiene (2,1-b:3,4-b') dithiophene to a 250 mL three-necked flask, then add 120 mL of a mixture of chloroform and acetic acid with a volume ratio of 1:1, and stir . Slowly add 0.20 mol of N-bromosuccinamide (NBS) to it, and after refluxing the reactant at 100°C for 3 hours, pour the reaction solution into cold water, separate the organic phase, extract the aqueous phase twice with chloroform, and combine The organic phase was washed twice with water, dried with anhydrous magnesium sulfate, and then the solvent was removed by rotary evaporation; the remaining product was separated by silica gel column chromatography to obtain cyclopentadiene (2,1-b:3,4-b') bis(5 -bromothiophene).
[0046] Step (2): Add 0.10 mol of cyclopentadiene (2,1-b:3,4-b') bis(5-bromothiophene) and 60 mL of tetrahydrofuran (THF) into a dry flask protected by argon,...
Embodiment 2
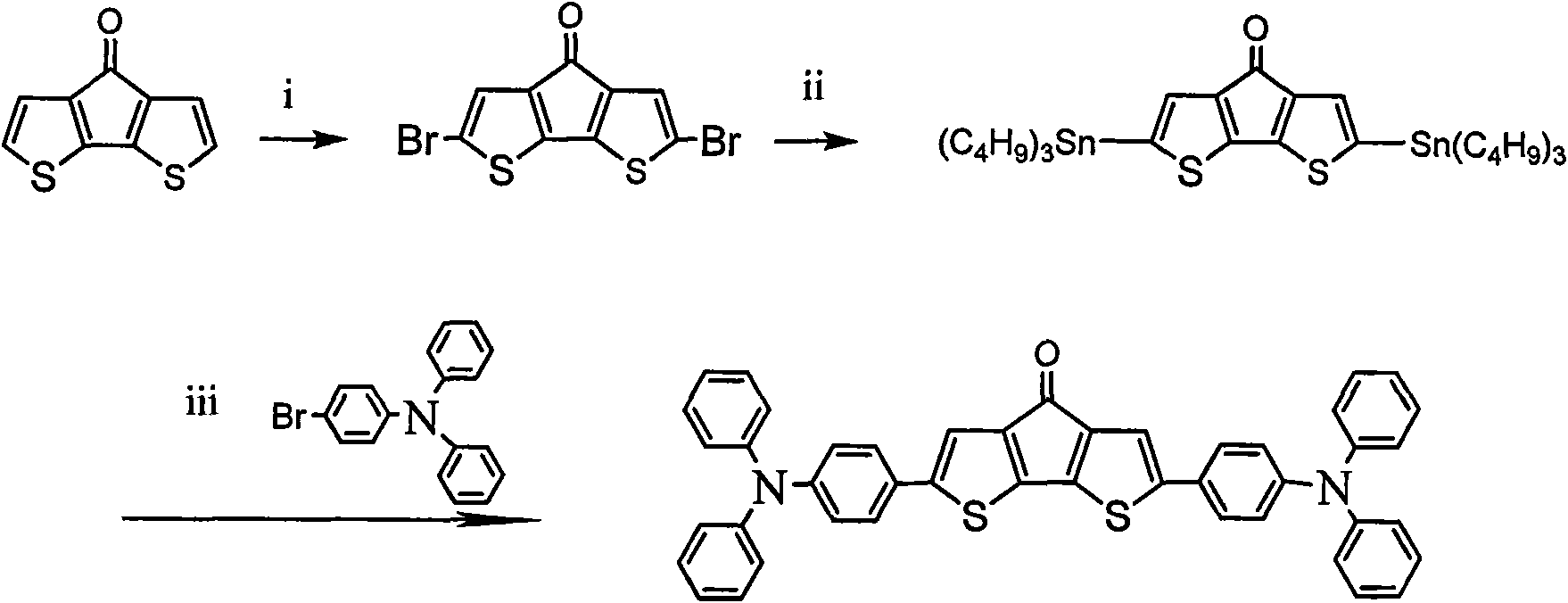
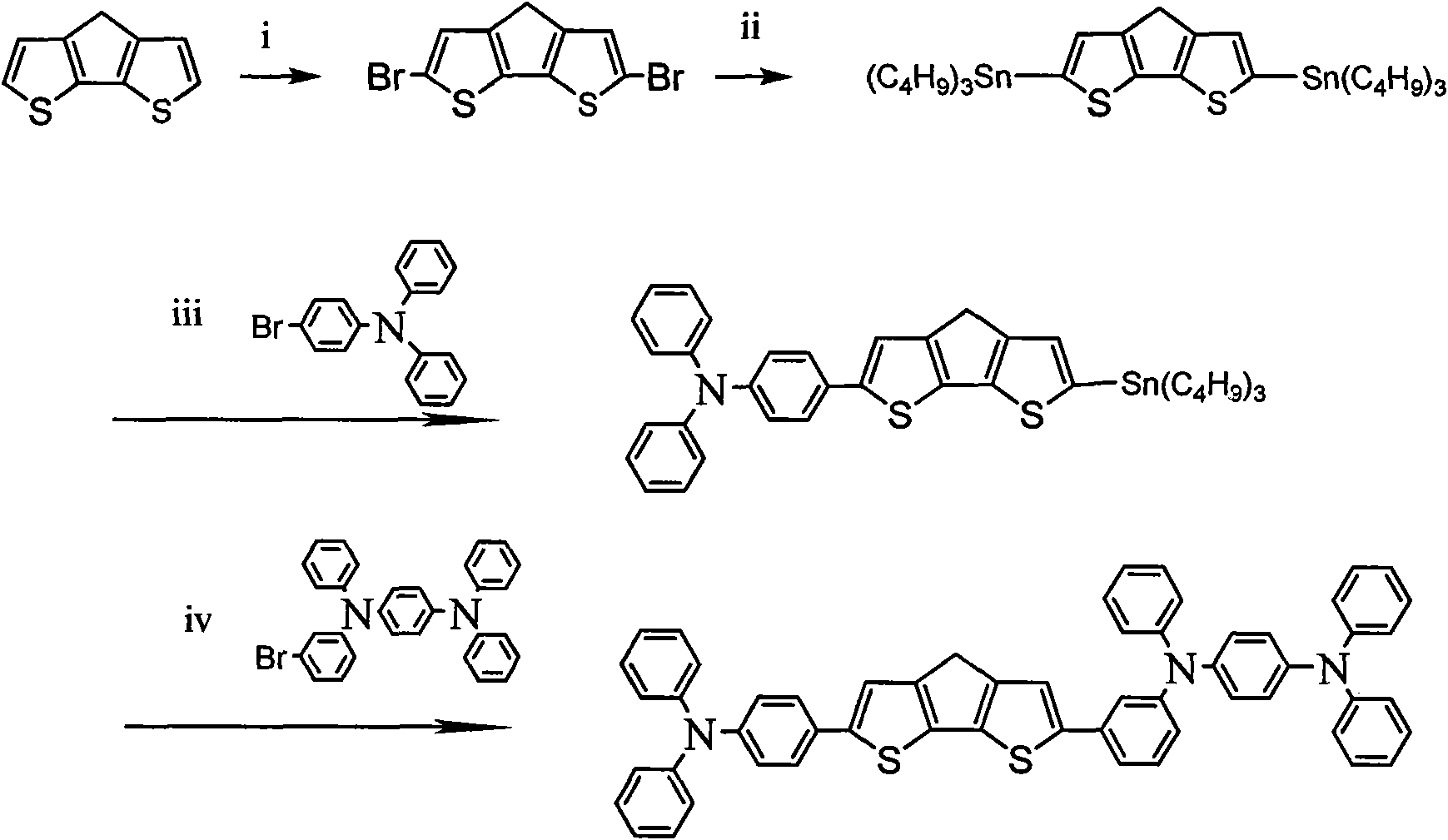
[0048] Example 2: N, N, N', N'-tetraphenyl-benzene-1,4-diamine-structure a-N,N,N',N'-tetraphenyl-benzene-1,4-diamine Preparation of:
[0049] Using the same method as in Example 1, replace the raw material N, N-diphenyl-N-4-bromophenylamine in step (3) with N, N, N'-triphenyl-N'-4 -Bromophenyl-1,4-phenylenediamine, obtain organic small molecule optoelectronic material N, N, N', N'-tetraphenyl-benzene-1,4-diamine-structure a-N, N, N', N'-tetraphenyl-benzene-1,4-diamine.
Embodiment 3
[0051] Using the same method as in Example 1, replace the raw material N, N-diphenyl-N-4-bromophenylamine in step (3) with N, N, N'-triphenyl-N'-4 -Bromophenyl-1,1'-biphenyl-4,4'-diamine, organic small molecule optoelectronic material N,N,N',N'-tetraphenyl-1,1'-biphenyl- 4,4'-Diamine-Structure a-N,N,N',N'-tetraphenyl-1,1'-biphenyl-4,4'-diamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com