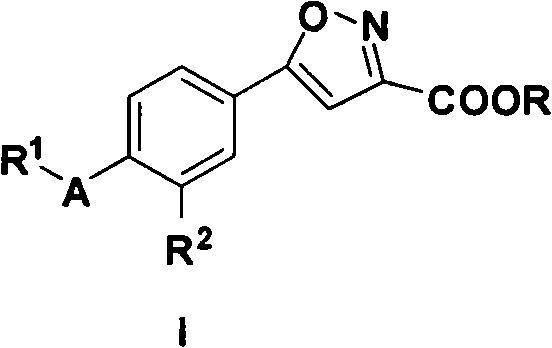
5-substituted phenyl-3-isoxazole carboxylic acid and ester compounds, compositions and preparation method thereof
A technology of ethyl isoxazolecarboxylate and isoxazolecarboxylate, which is applied in the field of medical technology, can solve the problem of not finding a preparation method for 5-substituted phenyl-3-isoxazolecarboxylate and its ester compound, etc., and achieves Effect of prevention of hyperuricemia and gout, simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of 5-(3-nitro-4-isobutoxy)phenyl-3-isoxazolecarboxylic acid
[0064] Prepare the title compound according to the following procedure
[0065]
[0066] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0067] Dissolve p-hydroxyacetophenone (15.00g, 110.00mmol) in glacial acetic acid (40mL), stir at 45℃, slowly add concentrated nitric acid (10.5mL, 110.00mmol) dropwise, control the reaction temperature at 50℃-60℃, After dripping, continue to stir for 0.5h. The reaction solution was poured into a mixture of ice and water, a yellow solid precipitated, filtered with suction, dried naturally at room temperature, and then recrystallized with absolute ethanol to obtain 10.50 g of yellow crystals with a yield of 52.6%, m.p. 131.0-132.6°C.
[0068] (2) Preparation of 3-nitro-4-isobutoxyacetophenone
[0069] Add 3-nitro-4-hydroxyacetophenone (15.80g, 87.30mmol), anhydrous potassium carbonate (42.20g, 306.00mmol), DMF (150.0mL), PEG-400 (4.0mL) to 250mL round bottom In t...
Embodiment 2
[0084] Preparation of 5-(3-nitro-4-benzyloxy)phenyl-3-isoxazolecarboxylic acid
[0085] Prepare the title compound according to the following procedure
[0086]
[0087] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0088] For specific operations, refer to Example 1 (1)
[0089] (2) Preparation of 3-nitro-4-benzyloxyacetophenone
[0090] In a 250ml round bottom flask was added 3-nitro-4-hydroxyacetophenone (5.00g, 27.60mmol), anhydrous potassium carbonate (11.40g, 82.90mmol), potassium iodide (0.27g, 1.38mmol) and DMF (50mL ), after stirring for 20 min at 65° C., benzyl chloride (6.4 mL, 55.20 mmol) was added, and the reaction was continued at this temperature for 3 h. Then, the reaction solution was poured into water (300 mL) to separate out solids, and filtered with suction to obtain a crude product, which was recrystallized from ethyl acetate to obtain 5.00 g of pale yellow crystals, with a yield of 66.8%, m.p. 132.7-134.7°C.
[0091] (3) Preparation of 4-(3-nitro-4-benz...
Embodiment 3
[0101] Preparation of 5-[3-nitro-4-(4-methyl)benzyloxy]phenyl-3-isoxazolecarboxylic acid
[0102] Prepare the title compound according to the following procedure
[0103]
[0104] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0105] For specific operations, refer to Example 1 (1)
[0106] (2) Preparation of 3-nitro-4-(4-methyl)benzyloxyacetophenone
[0107] In a 250ml round bottom flask was added 3-nitro-4-hydroxyacetophenone (5.00g, 27.60mmol), anhydrous potassium carbonate (11.40g, 82.90mmol), potassium iodide (0.30g, 1.38mmol) and DMF (50mL ), after stirring for 20 min at 65° C., 4-methylchlorobenzyl (7.80 g, 55.20 mmol) was added, and the reaction was continued at this temperature for 3 h. Then the reaction solution was poured into water (300 mL) to separate out solids, and filtered with suction to obtain a crude product, which was recrystallized from ethyl acetate to obtain 5.20 g of yellow crystals, with a yield of 66.0%, m.p. 108.8-110.8°C.
[0108] 1 H-NMR(300MHz, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com