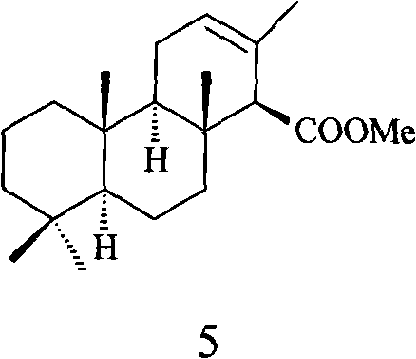
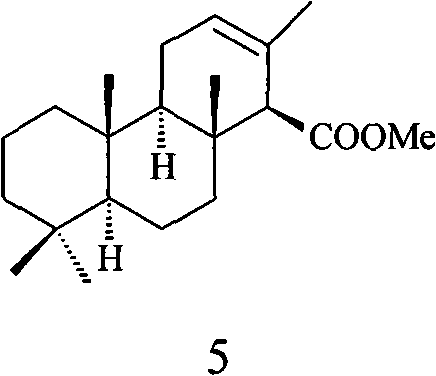
Method for preparing anomalous methyl ent-isocopalate
A technology of reaction and organic acid, applied in the field of preparation of tricyclic diterpenoids, can solve the problems of low total yield, lengthy synthesis route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
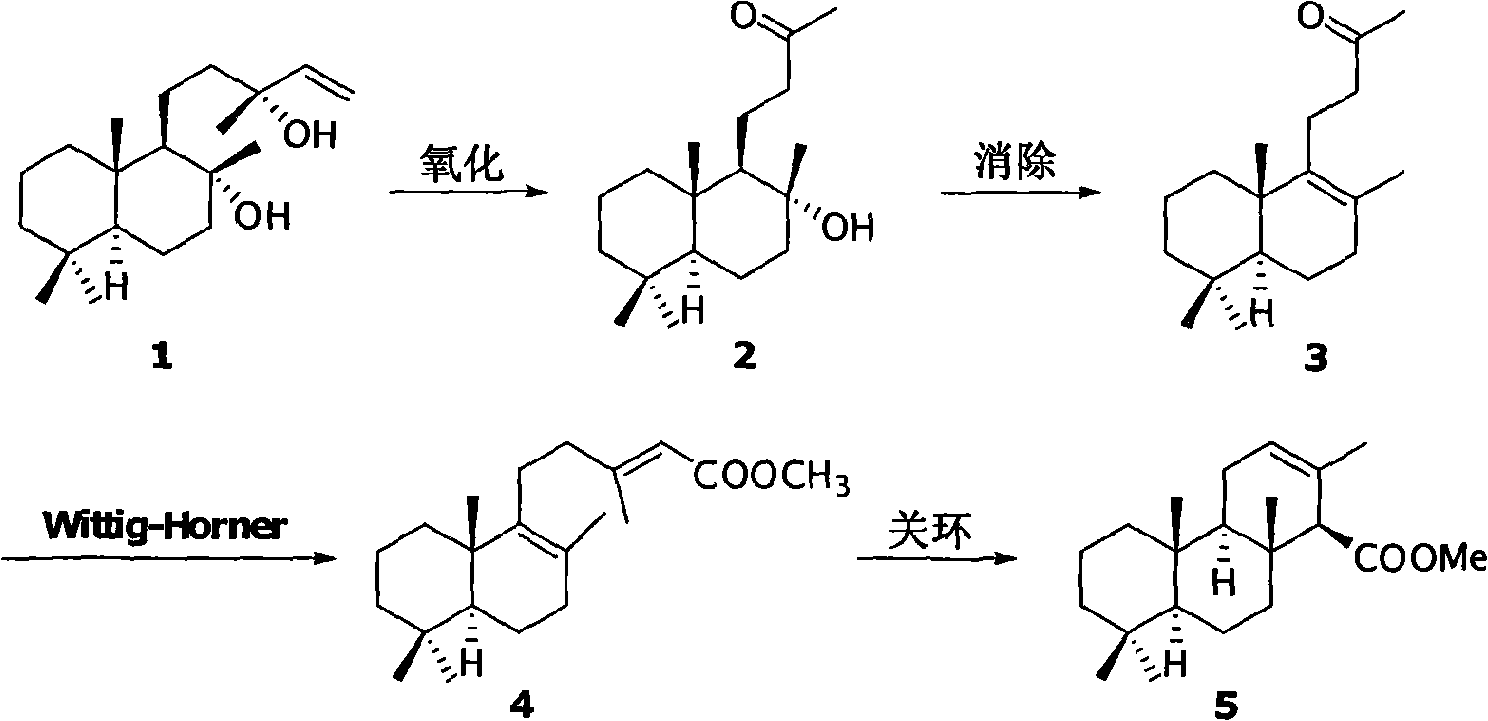
[0025] Preparation of Compound 2:
[0026]
[0027] Compound 1 (3.1g, 0.01mol) was dissolved in acetone (100ml) solution, potassium permanganate (7.9g, 0.05mol) and anhydrous sodium carbonate (8.5g, 0.08mol) were added in batches successively, and then Stirring was continued for 6 hours. After the reaction, the reaction solution was filtered through diatomaceous earth, the filter cake was washed with ethyl acetate, and the mother liquor was concentrated to obtain 2.5 g of a white solid crude product with a yield of 88%.
[0028] The crude product can be directly used in the next reaction without purification; it can also be recrystallized and purified with the following single or mixed solvents. The recrystallization solvent can be: a single solvent or a mixed solvent of petroleum ether, ether, n-hexane, cyclohexane, acetone, ethyl acetate, methylene chloride, chloroform, benzene or toluene.
[0029] Preparation of compound 3:
[0030]
[0031] Compound 2 (2.8g, 0.01m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com