Genetic liquid phase chip for joint detection of five drastic pathogenic bacteria and detection method thereof
A detection method and a joint detection technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganism determination/inspection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
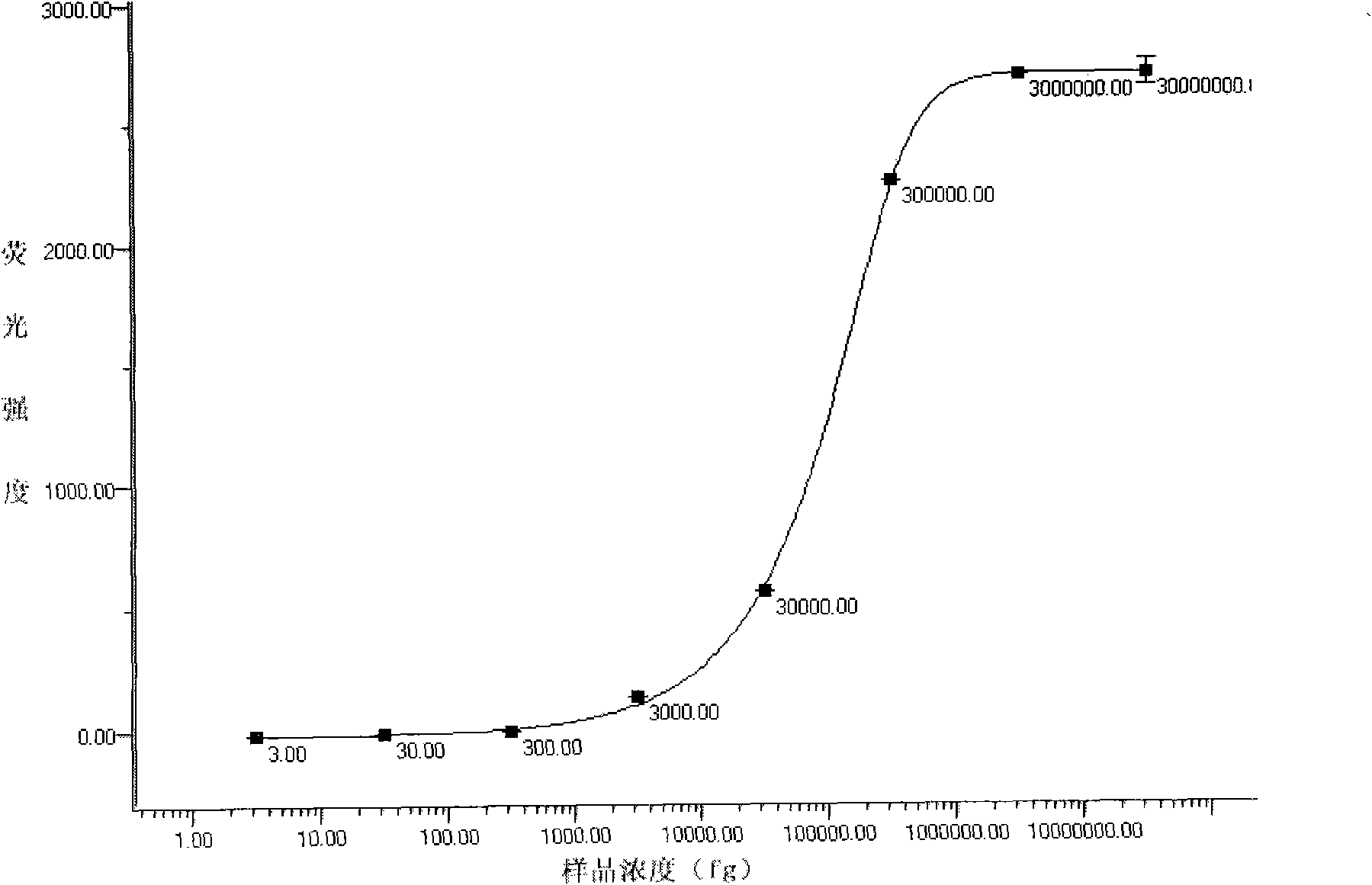
Image
Examples
Embodiment 1
[0057] Embodiment 1: adopt the multiplex PCR reaction of five kinds of bacteria six pairs of primers
[0058] 1. Extraction of nucleic acid in the sample: NaI cracking-glass powder adsorption method to extract nucleic acid.
[0059] 2. Prepare a multiplex PCR reaction system according to the following system, with a total volume of 30 μl:
[0060] 10×PCR buffer: 3μl
[0061] Taq E: 0.4 μl
[0062] dNTPs: 0.6 μl
[0063] BA-1-F, BA-1-R (10∏M): 0.3∏l each
[0064] BA-2-F, BA-2-R (10∏M): 0.3∏l each
[0065] YP-F, YP-R(10∏M): 0.3∏l each
[0066] Bru-F, Bru-P (10∏M): 0.36∏l each
[0067] FT-F, FT-R (10∏M): 0.24∏l each
[0068] BP-F, BP-R(10∏M): 0.24∏l each
[0069] Template DNA: 2∏l
[0070] wxya 2 O: add to 30∏l
[0071] 3. Carry out PCR reaction according to the following reaction conditions
[0072] Pre-denaturation:
[0073] 94°C 10 minutes
[0074] 32 loops:
[0075] 94°C for 30 seconds
[0076] 58°C 30 seconds
[0077] 72°C 40 seconds
[0078] extend:
[00...
Embodiment 2
[0082] Example 2: Coupling of capture probes to microspheres
[0083] 1. Select coded microspheres No. 044, 042, 032, 025, 034, and 027 respectively, and oscillate the microsphere suspension with a vortex oscillator to mix the microspheres evenly.
[0084] 2. Take the above microspheres about 1.25×10 6 Each was transferred to a centrifuge tube, centrifuged at 14000g for 3-5min, and the supernatant was carefully aspirated.
[0085] 3. Add 50∏l 0.1mol / L 2-(n-morpholino)ethanesulfonic acid solution, vibrate for 20-30s, and sonicate for 20-30s to resuspend the microspheres.
[0086] 4. Dilute the synthesized oligonucleotide probe to 0.1 mmol / L with distilled water.
[0087] 5. Add the 1∏l diluted probe to the microsphere suspension, and add the anthrax probe 1, probe 2, and Yersinia pestis probe corresponding to No. Needle, Brucella probe, Tularemia probe, melioidosis probe, shake and mix.
[0088] 6. Add 2.5∏l freshly prepared 10mg / mL EDC solution to the microsphere and probe...
Embodiment 3
[0097] Example 3: Detection of samples to be tested with the liquid-phase chip of the above-mentioned five kinds of virulent pathogenic bacteria
[0098] 1. Take 3500 of each of the above-mentioned coupled microspheres and mix them, and distribute them in PCR tubes (calculate the corresponding addition amount according to the counting results of the microspheres)
[0099] 2. Add 5-17∏l of the PCR products of the five bacterial mixed templates to each tube to make the final volume 50∏l, and mix by pipetting.
[0100] Denature at 3.95°C for 10 minutes.
[0101] 4. React at 45-60°C for 10-30 minutes.
[0102] 5. Transfer to a 96-well filter plate and filter to remove unbound PCR products.
[0103] 6. Add 75∏l4ng / ∏l SA-PE to each well, incubate at room temperature in the dark for 10 minutes, and remove unbound SA-PE by suction filtration.
[0104] 7. Add 75∏l of the suspension to each well and shake to resuspend the microspheres.
[0105] 8. Check on the machine after the reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com