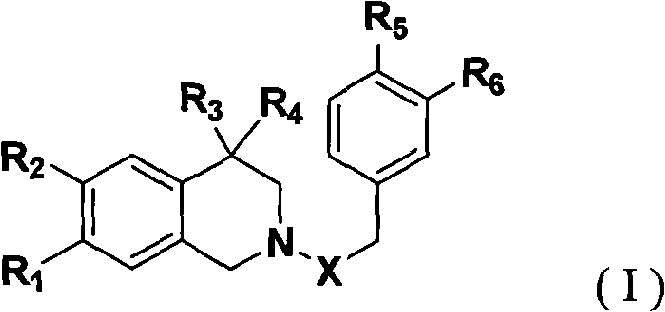
1, 2, 3, 4-tetrahydroisoquinoline derivatives and synthetic method and uses thereof
A technology of tetrahydroisoquinoline and derivatives, applied in 1 field, can solve the problems of high toxicity and slight curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
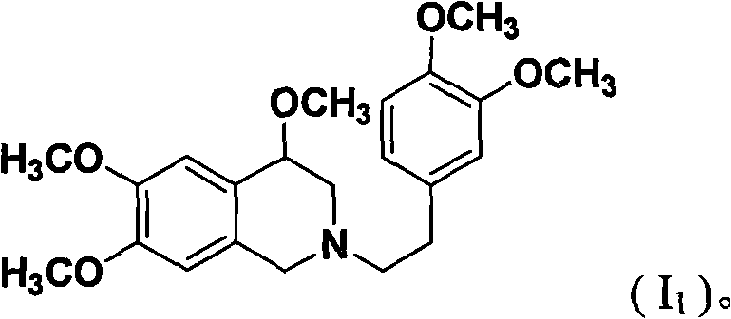
[0041] 2-(3',4'-dimethoxyphenylacetyl)-4,7,8-trimethoxy-1,2,3,4-tetrahydroisoquinoline (VI 1 ) preparation
[0042] (1) Preparation of 3,4-dimethoxy-β-nitrostyrene (II)
[0043] Dissolve 16.60 g of 3,4-dimethoxybenzaldehyde in 20 mL of methanol, add 6.10 g of nitromethane, and slowly add 10 mL of cooled NaOH solution (10.5 mmol / mL) dropwise in an ice bath, and a large amount of white solids appear. Pour into 60mL of ice water to dissolve, and put it in an ice bath to cool down. At 5°C, pour it into 60mL of hydrochloric acid (1:1) under vigorous stirring, and a large amount of yellow precipitate precipitates out. Suction filtration, drying, recrystallization from absolute ethanol, and vacuum drying gave yellow crystals with a yield of 88.6% and a melting point of 141.2-142.0°C.
[0044] (2) 1,2-dimethoxy-4-(2-nitro-1-methoxyethyl)benzene (III 1 ) preparation
[0045] Dissolve 8.42g of 3,4-dimethoxy-β-nitrostyrene (II) in 80ml of dry diethyl ether and mix, cool in an ice bat...
Embodiment 2
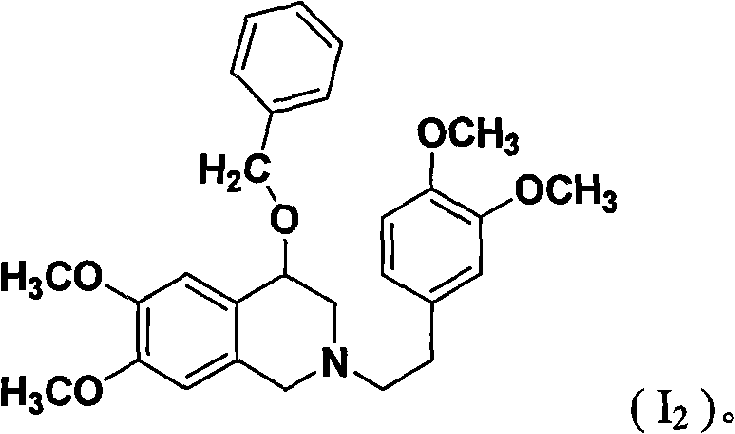
[0055] 2-(3',4'-dimethoxyphenylacetyl)-4-benzyloxy-7,8-trimethoxy-1,2,3,4-tetrahydroisoquinoline (VI 2 ) preparation
[0056] (1) 1,2-dimethoxy-4-(2-nitro-1-benzyloxyethyl)benzene (III 2 ) preparation
[0057] Mix 80 mL of ether solution of 8.25 g of 3,4-dimethoxy-β-nitrostyrene (II) with ether suspension (40 mL) of newly prepared sodium benzyl alcohol (160 mmol), and press III 1 During the preparation process, yellow crystals were obtained, yield: 75.8%, melting point: 77.2-78.6°C.
[0058] (2) 2-(3,4-dimethoxyphenyl)-2-benzyloxyethylamine (VI 2 ) preparation
[0059] 6.34g of 1,2-dimethoxy-4-(2-nitro-1-benzyloxyethyl)benzene (III 2 ) in anhydrous tetrahydrofuran solution 40mL was added dropwise to 3.92g lithium aluminum hydride in anhydrous tetrahydrofuran solution (80mL), according to IV 1 After treatment in the preparation process, an orange-yellow oil was obtained, the yield: 70.5%, and the product was directly put into the next step without purification.
[0060] ...
Embodiment 3
[0067] 2-(3',4'-dimethoxyphenylacetyl)-4-cyclohexyloxy-7,8-trimethoxy-1,2,3,4-tetrahydroisoquinoline (VI 3 ) preparation
[0068] (1) 1,2-dimethoxy-4-(2-nitro-1-cyclohexyloxyethyl)benzene (III 3 ) preparation
[0069] Put 8.09g of 3,4-dimethoxy-β-nitrostyrene (II), newly prepared sodium cyclohexylate (160mmol) and 100mL of dioxane 100mL into a 250mL three-necked bottle, and force Stir the reaction, trace with thin-layer chromatography (TLC), after the reaction is complete, add 10.5mL of glacial acetic acid dropwise to produce a light yellow viscous liquid, spin the solvent, add 30mL of water, extract the aqueous phase with 30mL×3 methylene chloride, anhydrous sulfuric acid Dried over magnesium, spin-dried the solvent, and distilled under reduced pressure to obtain a brownish-yellow oil, yield: 45.8%.
[0070] (2) 2-(3,4-dimethoxyphenyl)-2-cyclohexyloxyethylamine (IV 3 ) preparation
[0071] 6.20g of 1,2-dimethoxy-4-(2-nitro-1-cyclohexyloxyethyl)benzene (III 3 ) of 40mL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com