Oral solid pharmaceutical composition containing clinofibrate
A composition and drug technology, which is applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as Clibite hydrophobicity, and achieve good reproducibility, simple process, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
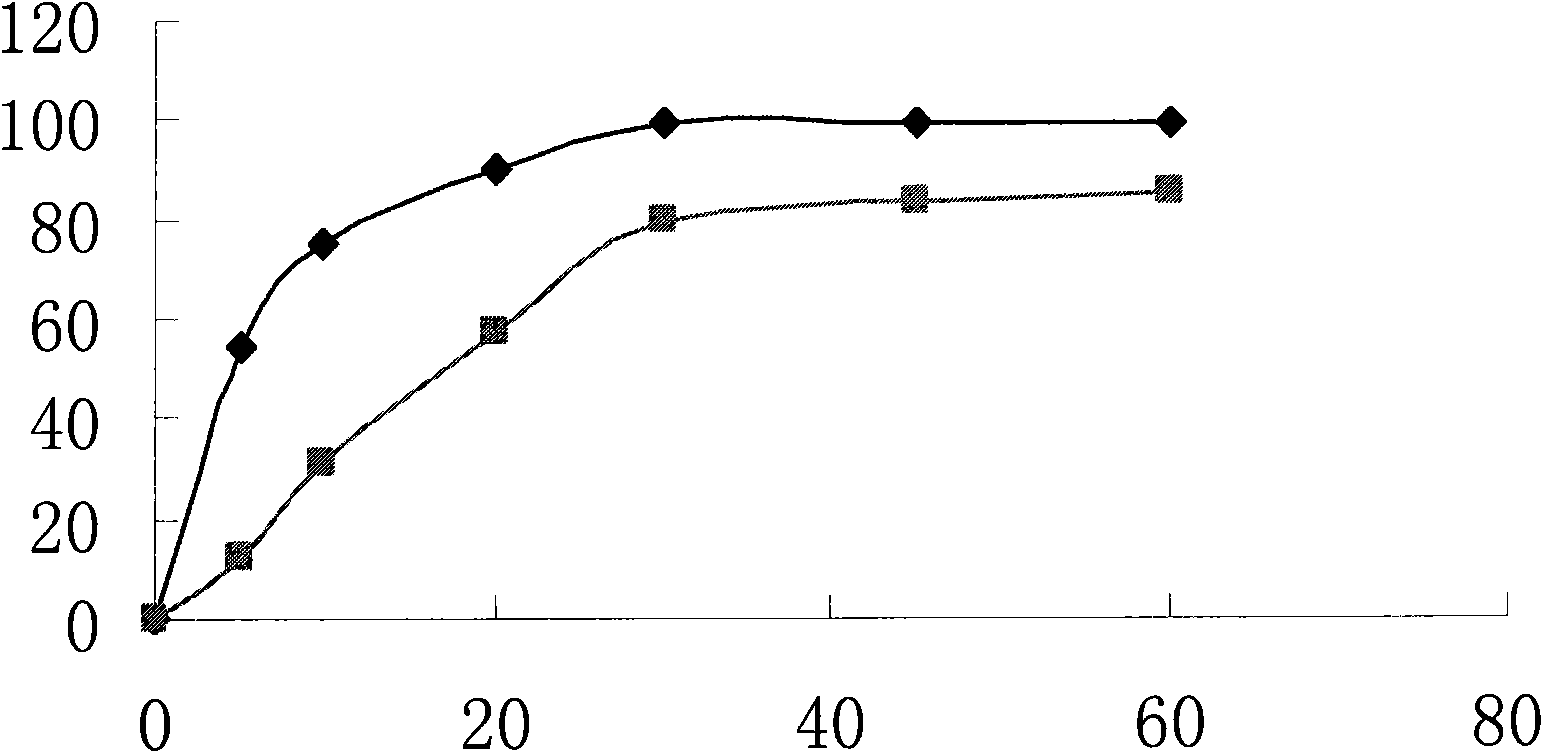
Image
Examples
Embodiment 1
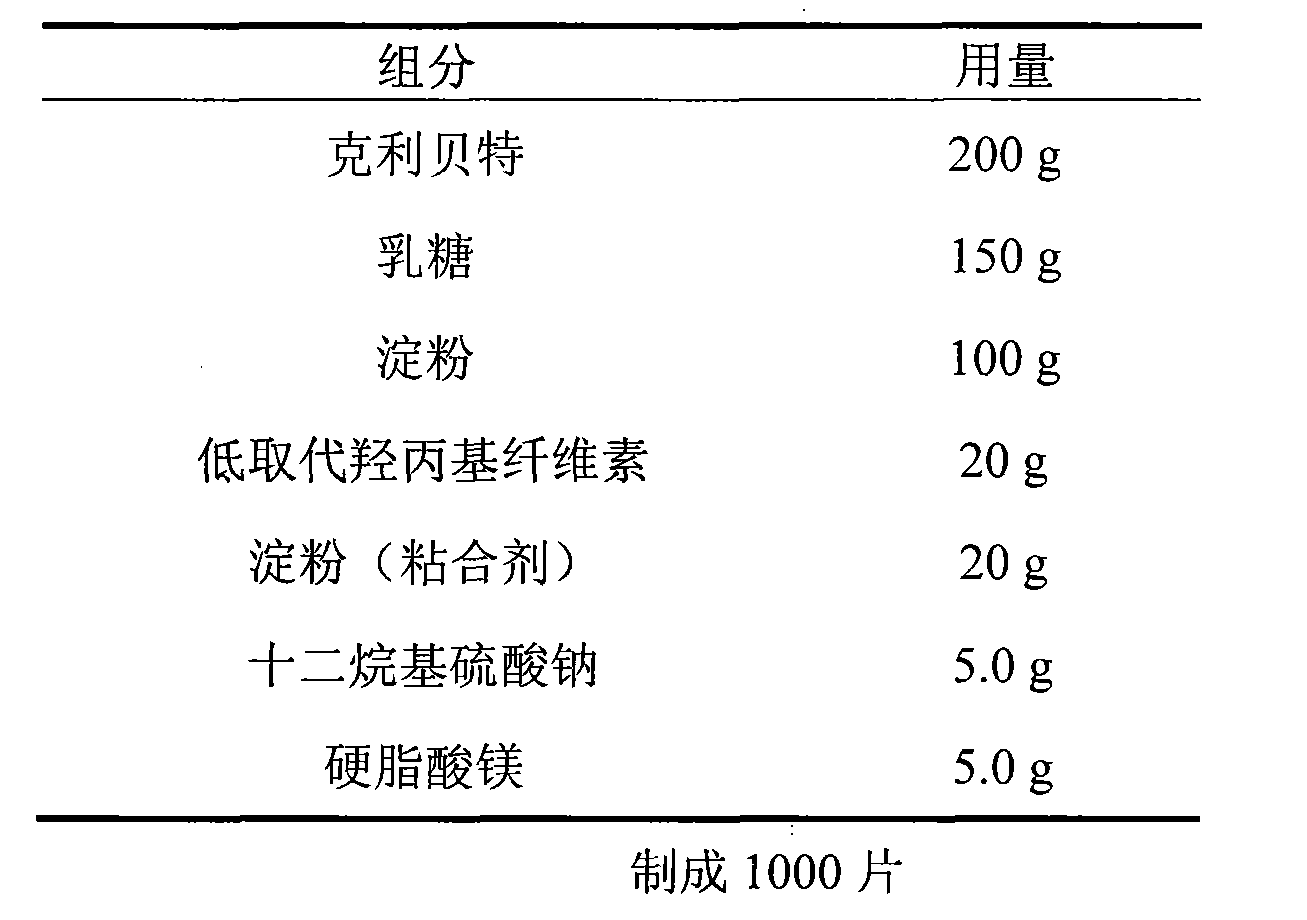
[0015] Embodiment 1 (the existence form of this embodiment is tablet)
[0016]
[0017] Preparation:
[0018] The starch in the recipe amount is prepared into starch slurry, and sodium lauryl sulfate is added and stirred evenly as a binder. The binder is added into the mixed material, granulated, dried, added with magnesium stearate, and pressed into tablets to obtain the finished product.
Embodiment 2
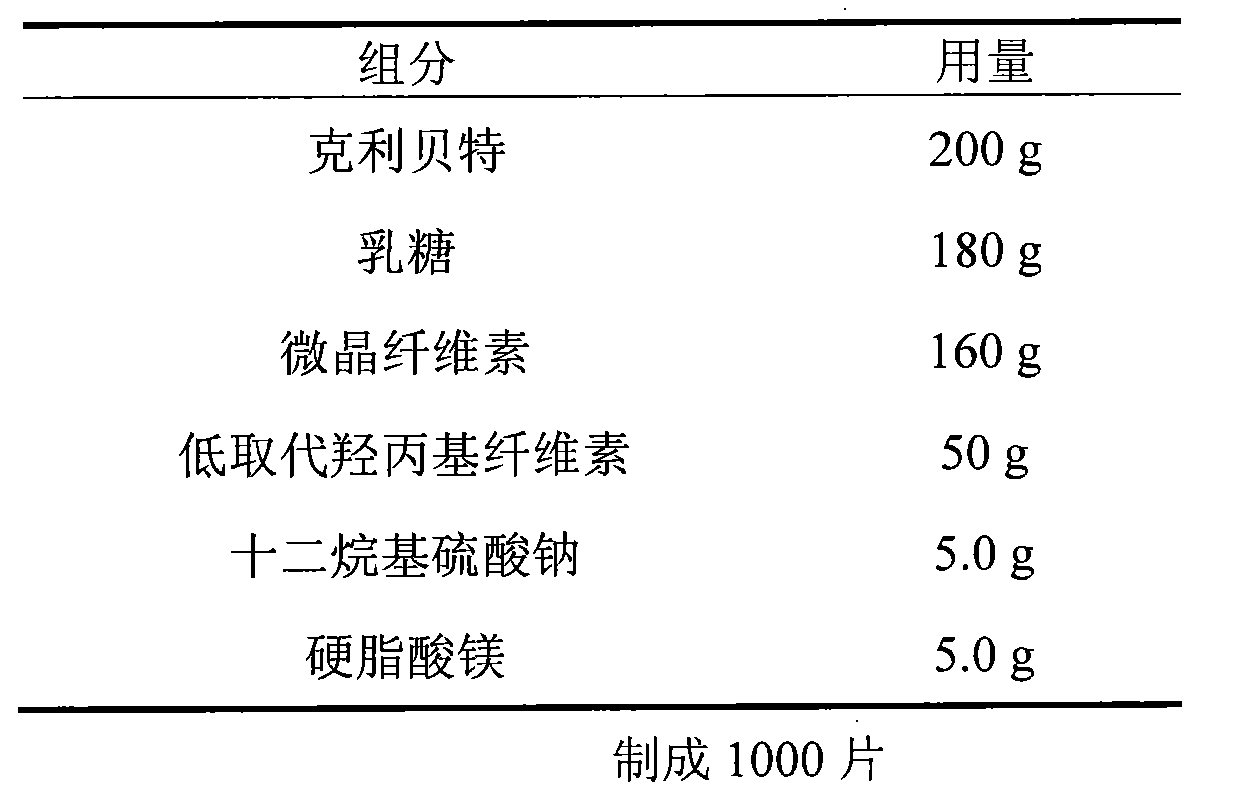
[0019] Embodiment 2 (the existence form of this embodiment is dispersible tablet)
[0020]
[0021] Preparation:
[0022] Add the prescribed amount of sodium lauryl sulfate into purified water, stir to dissolve, and act as a wetting agent. The binder is added to the mixed material, granulated, dried, added with magnesium stearate and an additional part of the disintegrating agent low-substituted hydroxypropyl cellulose, and compressed into tablets to obtain the finished product.
Embodiment 3
[0023] Embodiment 3 (the existence form of this embodiment is capsule)
[0024]
[0025] Preparation:
[0026] Add the prescribed amount of sodium lauryl sulfate into purified water, stir to dissolve, and act as a wetting agent. The binder is added to the mixed material, granulated, dried, added with micropowdered silica gel, packed into a capsule shell, and obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com