Preparation of recombinant protein of human vascular endothelial cell growth inhibition factor rhVEGI-192A
A technology of VEGI-192A and growth inhibitor, which is applied in the biological field, can solve the problems of low cost and lack of high efficiency, and achieve the effects of reducing production costs, optimizing expression conditions, and increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Construction of recombinant protein rhVEGI-192A expression vector
[0038]The rhVEGI-192A target gene was amplified from total RNA of human umbilical vein endothelial cells by PCR. The upstream primer of PCR is 5'-TTCCATATGCAACTCACAAAAGGGCCGTCT-3', and the downstream primer is: 5'-CGCGGATCCCTATAGTAAGAAGGCTCCAAAGAAGGTT-3'. Both upstream and downstream primers contain Nde I restriction enzyme cutting sites at the 5' end and BamH I restriction enzyme cutting sites at the 3' end. Then use the above two restriction endonucleases to cut out the target gene, and finally connect it to the expression vector pET-30a to construct the recombinant protein rhVEGI-192A expression vector. The 3' end of the insertion site contains a nucleotide sequence encoding 6 histidines. The accuracy and correct reading frame of the rhVEGI-192A recombinant were confirmed by sequence determination.
Embodiment 2
[0039] Example 2: IPTG-induced expression of rhVEGI-192A in Escherichia coli and screening of highly expressed clones.
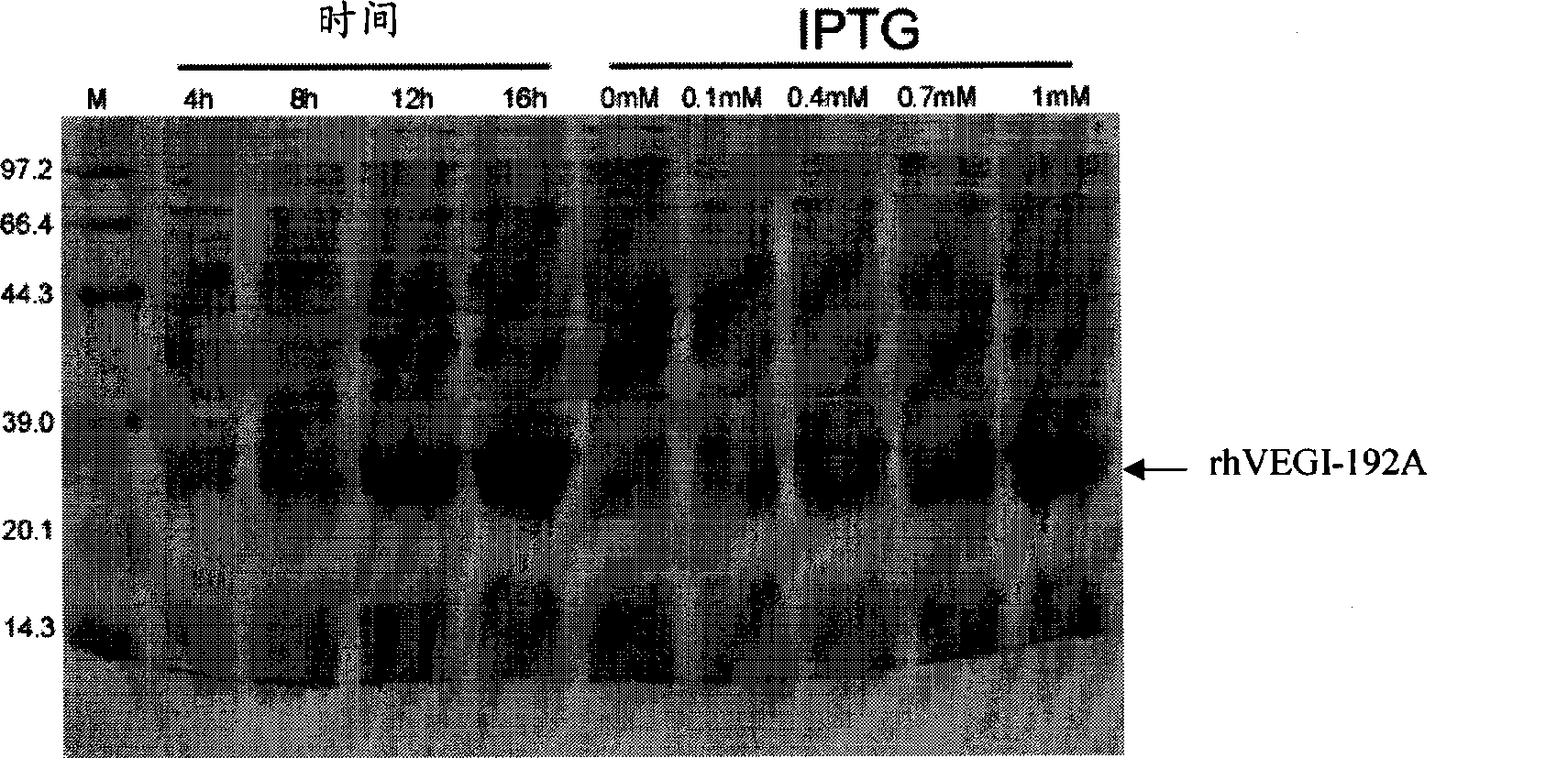
[0040] 1. IPTG induction: Use the expression vector pET-30a-rhVEGI-192A to transform the competent cell BL21(DE3)pLysS, randomly pick 16 clones and colonize them in 3 mL LB medium, add kanamycin to a final concentration of 50 μg / ml , 37 ° C recovery culture overnight. The next day, measure the density of each tube of bacterial liquid. If the OD600 is between 0.6 and 1, add IPTG to a certain final concentration, and induce culture for 4 hours. The negative control is induced without adding IPTG. The recombinant expression was analyzed by SDS-PAGE. 183 clonal colonies were obtained.
[0041] 2. LB medium: 1% peptone, 0.5% yeast extract, 1% sodium chloride, pH7.0; carbenicillin: 50mg / ml; IPTG solution: 100mmol / L;
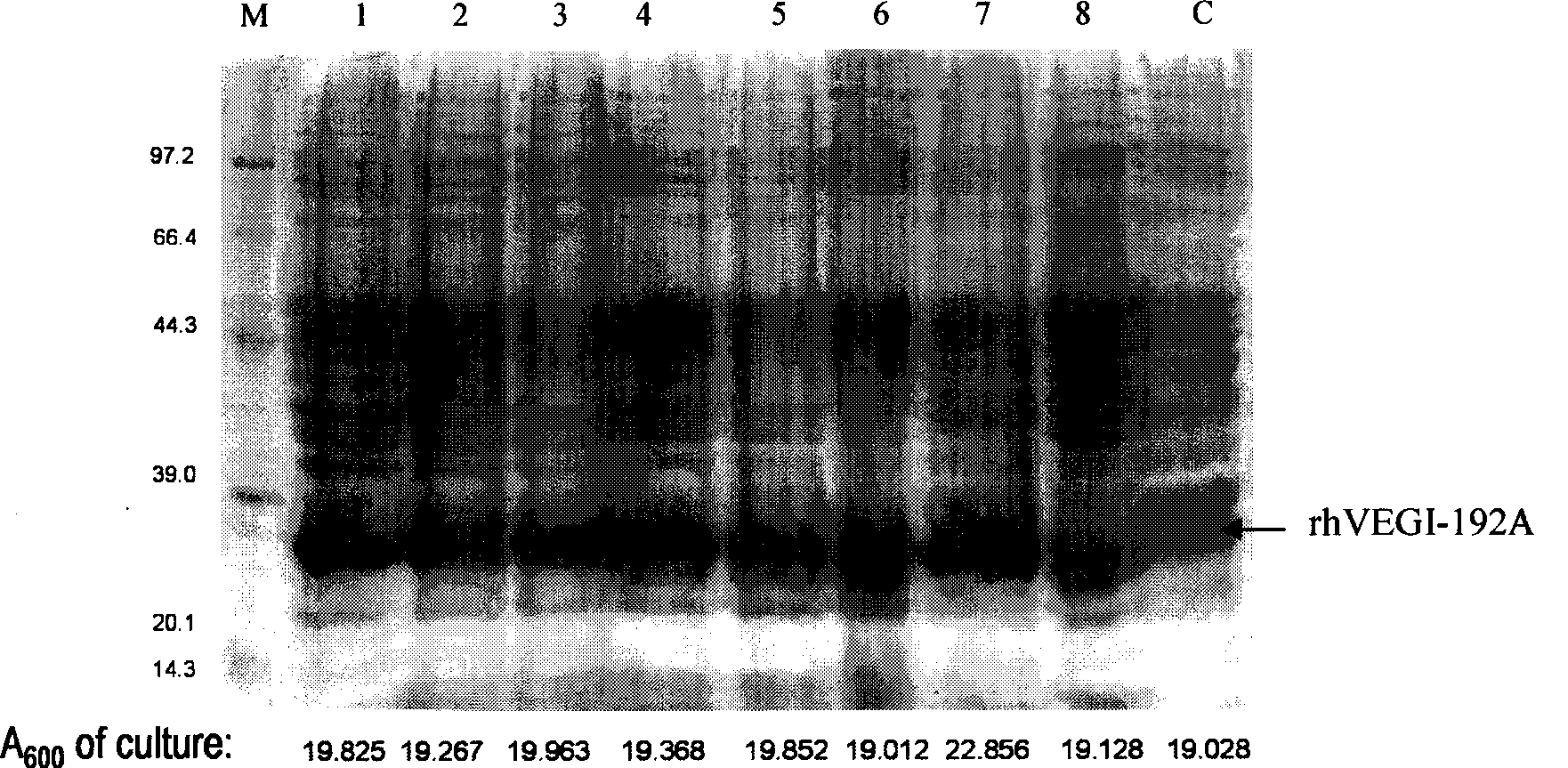
[0042] 3. Screening high-expression clones of rhVEGI-192A protein: Using high-throughput SDS-PAGE method, 16 single colonies with the highest exp...
Embodiment 3
[0044] Example 3: Condition optimization of IPTG-induced expression system
[0045] Different culture conditions affect the expression of the target protein, including temperature, IPTG concentration, density of engineered bacteria during induction (OD600 value), host bacteria, induction time and induction system. The optimal expression conditions are explored through preliminary experiments. The #3 clone colony was selected for the optimization experiment of protein induction expression conditions. figure 2 It is the condition optimization of the IPTG-induced expression system of the present invention, including the culture time before bacterial induction and the amount of IPTG added, and the host bacteria is BL21(DE3)pLysS. Each group of screening changes a parameter. When screening the best colonies, the host bacteria used is BL21(DE3)pLysS, and the induction conditions are IPTG: 1mM, 16h, temperature: 25°C; when screening the best IPTG concentration, the host bacteria use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com