Process for production of optically active aminophosphinylbutanoic acid
一种氨基氧膦基丁酸、旋光性的技术,应用在旋光性氨基氧膦基丁酸类的制备领域,能够解决催化效率不高、铑催化剂昂贵、后处理和纯化步骤复杂等问题,达到高光学纯度、高效且光学纯度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
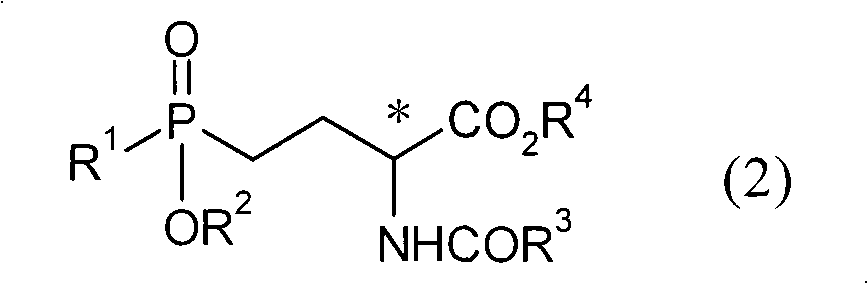
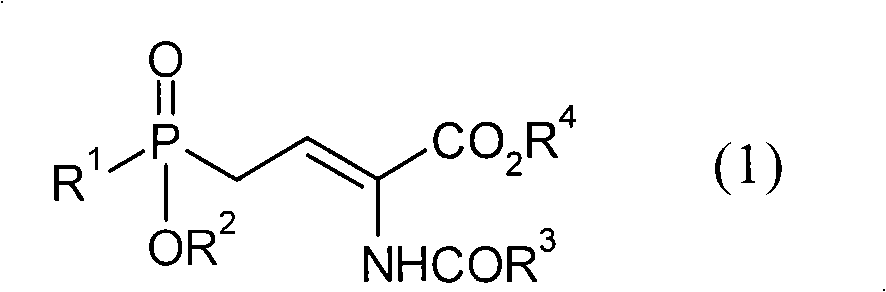
[0034] In the preparation method of the present invention, the optically active aminophosphine oxide represented by the above general formula (2) is obtained by asymmetric hydrogenation of the compound represented by the general formula (1) in the presence of a ruthenium-optical phosphine complex butyric acid.
[0035]
[0036] (In general formula (1), R 1Represents an alkyl group with 1 to 4 carbon atoms, R 2 Represents a hydrogen atom or an alkyl group with 1 to 4 carbon atoms, R 3 Represents an alkyl group having 1 to 4 carbon atoms, an alkoxy group having 1 to 4 carbon atoms, an aryl group, an aryloxy group or a benzyloxy group, R 4 represents a hydrogen atom or an alkyl group having 1 to 4 carbon atoms. )
[0037] For the compound represented by the general formula (1) used in the present invention and the optically active aminophosphinyl butyric acids represented by the general formula (2) prepared by the present invention, R 1 , R 2 , R 3 and R 4 group shown....
Embodiment 1
[0090] Preparation of (S)-2-Acetamido-4-Hydroxymethylphosphinylbutanoic Acid
[0091]
[0092] Add (Z)-2-acetamido-4-hydroxymethylphosphinyl-2-butenoic acid (4.0 g, 18 mmol) and (RuCl (p-cymene) (S) in a 200 ml autoclave -binap)Cl (8.4mg, 0.009mmol) and methanol (20ml), nitrogen substitution and hydrogen substitution were carried out. The temperature in the autoclave was set to 70° C., hydrogen gas was injected up to 1 MPa, and the reaction solution was stirred at the same temperature for 5 hours. Take a part of the reaction solution as a sample. After confirming the completion of the reaction by performing high performance liquid chromatography (HPLC) analysis on the sample, the reaction solution was cooled to room temperature, and after hydrogen gas was released, the reaction solution was transferred to a 100ml flask. After removal of methanol in vacuo and addition of water (20ml), the aqueous phase was washed twice with toluene (10ml) to give (S)-2-acetamido-4-hydroxym...
Embodiment 2~4
[0095] Preparation of (S)-2-Acetamido-4-Hydroxymethylphosphinylbutanoic Acid
[0096] Except that the amount of (RuCl (p-cymene) (S)-binap) Cl was doubled, and the reaction time and reaction temperature were changed as shown in Table 1, the same operation as in Example 1 was carried out. The results are shown in Table 1. Also, the conversion rates were all 100%.
[0097] (Table 1)
[0098] Example
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com