Recombinant adenovirus for expressing human CREG and uses thereof
A recombinant adenovirus and application technology, applied in the field of recombinant adenovirus, can solve the problems of lack of clinical application feasibility, destruction of host cell genome stability, and no feasibility of clinical drug use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] Embodiment: 1. Construction of the recombinant adenoviral vector carrying the full sequence of human CREG cDNA
[0033] Design the RT-PCR primers of CREG gene according to the CREG cDNA sequence given in GenBank (NM_003851), and the primer sequences are as follows:
[0034] Forward primer: 5`---aa ggatcc atg gcc ggg cta tcc cgc-3'
[0035]Reverse primer: 5′-gc gaattc tca ctg aac tgt gac att ata ata ttcttc tgg-3′
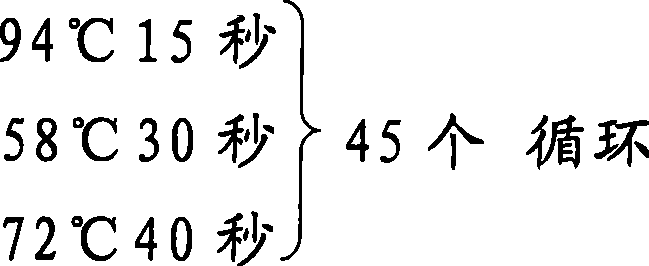
[0036] Specific conditions:
[0037] dH 2 O 36.5 μL
[0038] dNTP 4μL
[0039] Buffer 5 μL
[0040] Primer (1) 1.5 μL
[0041] Primer (2) 1 μL
[0042] Template 1 μL
[0043] DNA polymerase (Takara company) 1 μL
[0044]
[0045] The mRNA was extracted from VSMC cultured in vitro and reverse-transcribed into cDNA (cDNA first-strand synthesis kit from TakaRa Company). Amplification conditions were: 25°C for 5 minutes, 42°C for 50 minutes, and 95°C for 10 minutes. A total of 667 bases of the human CREG cDNA coding sequence was amplified by the foll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com