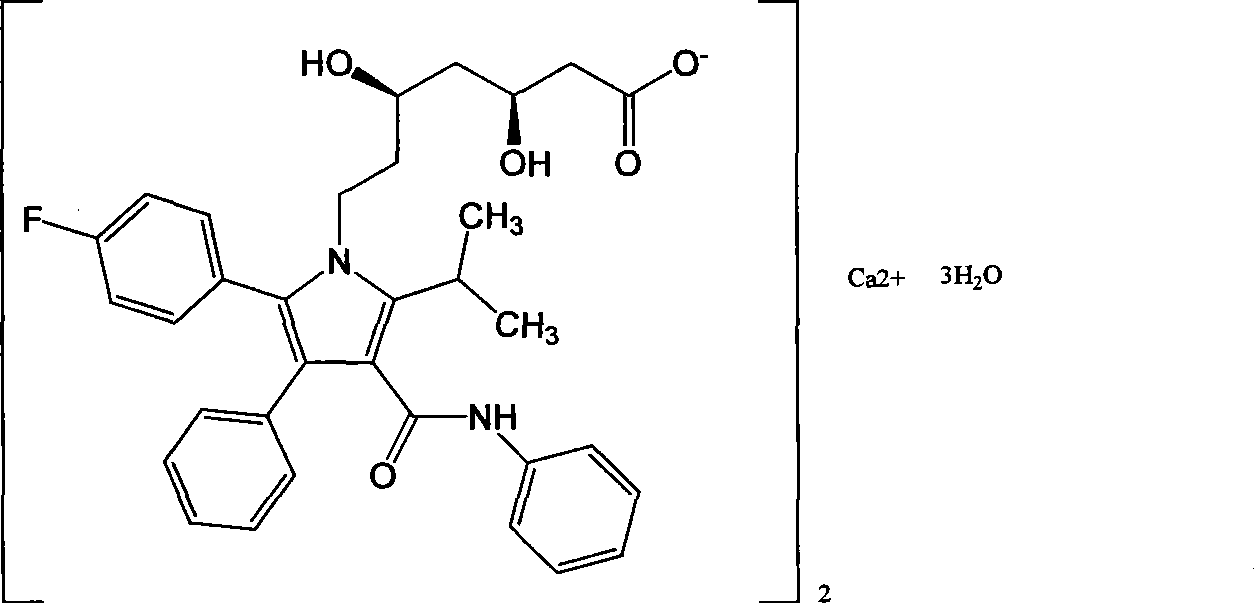
Preparation of amorphous atorvastatin calcium
An atorvastatin calcium and amorphous technology, which is applied in the new preparation field of amorphous atorvastatin calcium, can solve the problems of flammability and explosion, and achieves the advantages of less time-consuming filtration, reduced hidden dangers in safety production, and easy large-scale operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
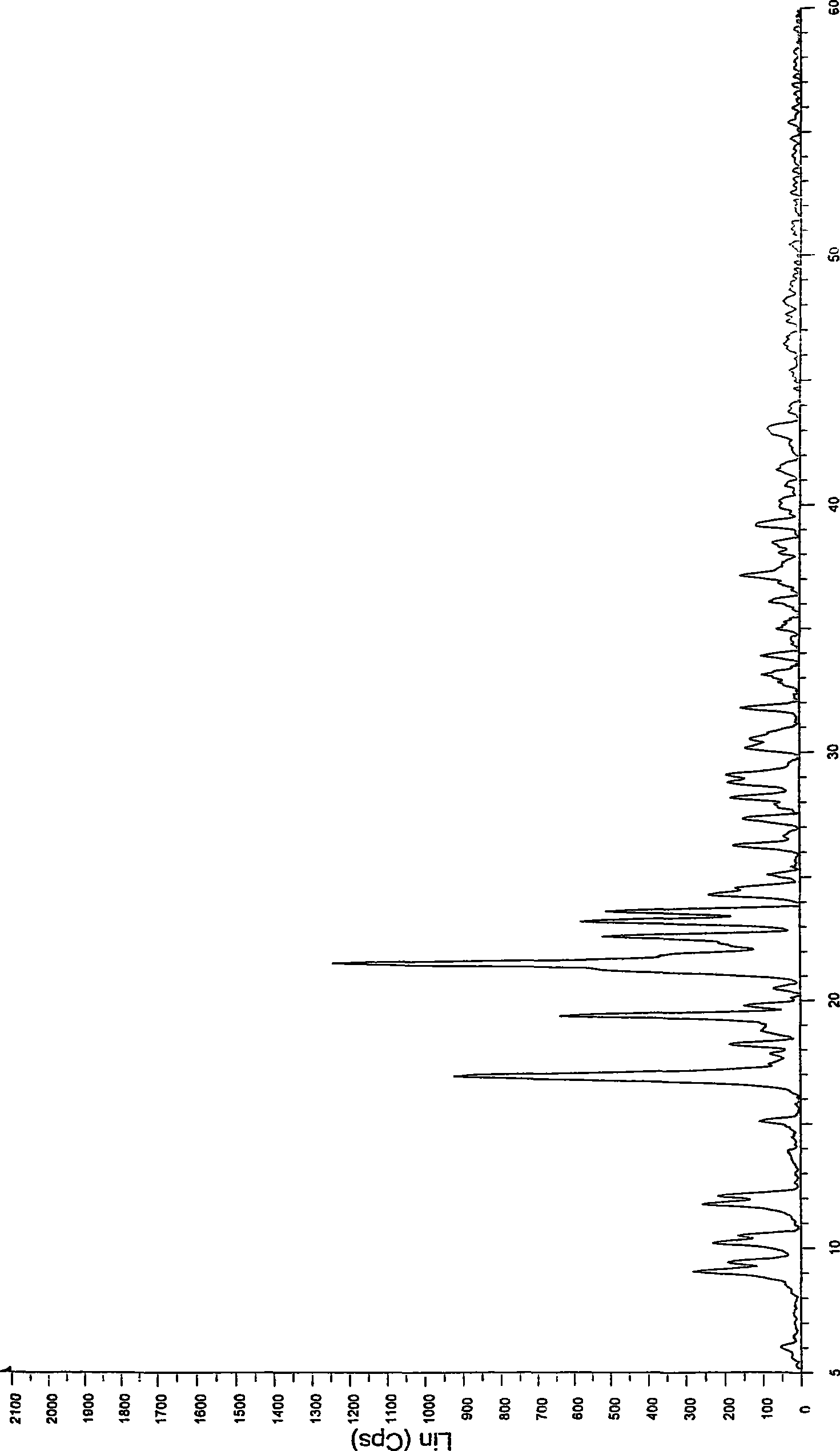
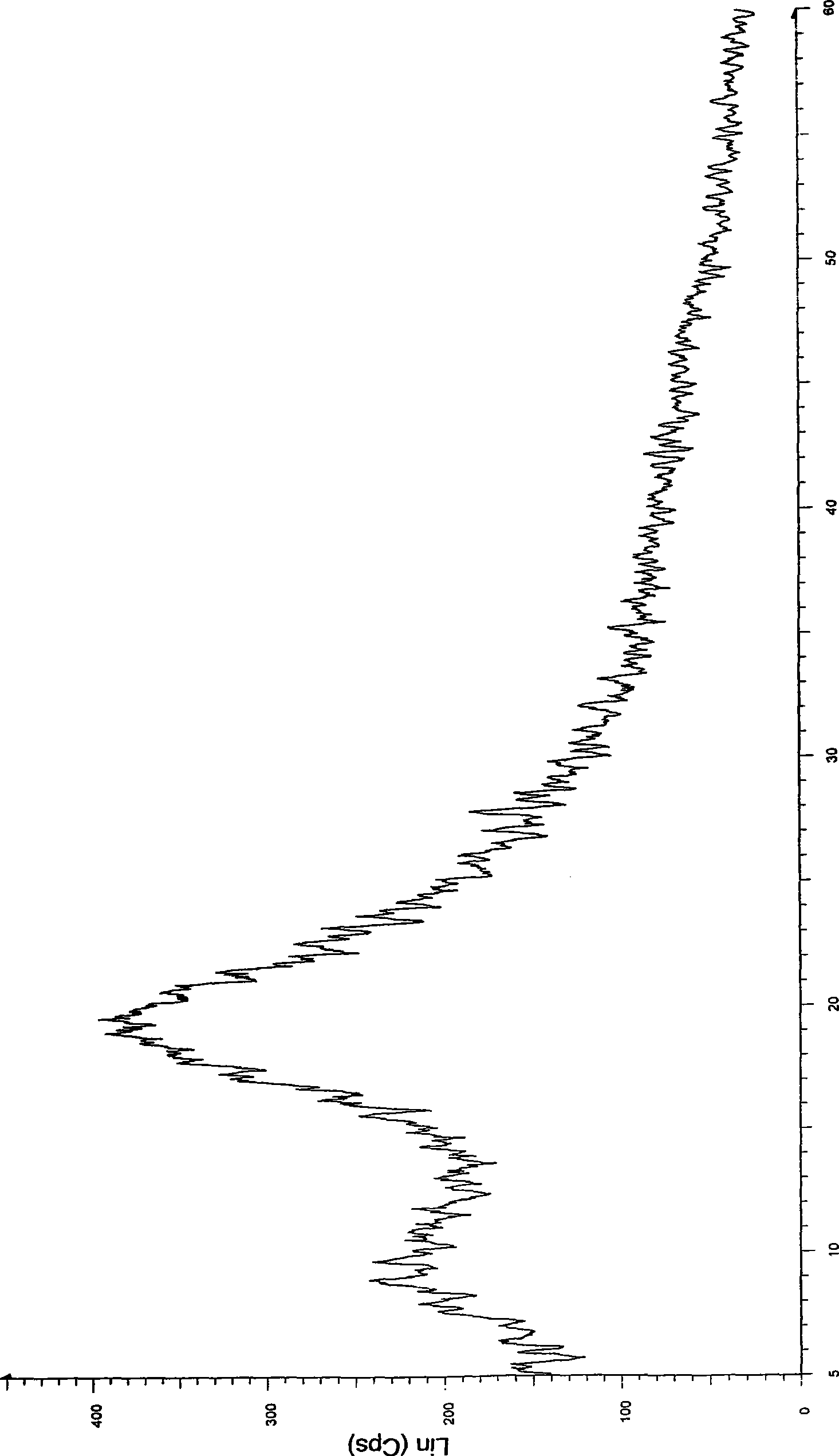
[0032] Take 1 kg of crystalline atorvastatin calcium, add 5 L of methanol, stir to dissolve, slowly add it into 40 L of isopropyl ether at room temperature, precipitate a solid, continue stirring for 2 hours, filter, drain, and vacuum dry at 50 ° C for 12 hours to obtain 850 g Amorphous atorvastatin calcium, X-ray powder diffraction pattern confirms the amorphous nature of the product.
Embodiment 2
[0034] Take 1kg of crystalline atorvastatin calcium, add 15L of ethanol, stir to dissolve, slowly add to 160L of isopropyl ether at room temperature, precipitate solid, continue to stir for 2 hours, vacuum filter, drain, and vacuum dry at 50°C for 12 hours to obtain 830 g of amorphous atorvastatin calcium, the X-ray powder diffraction pattern confirms the amorphous nature of the product.
Embodiment 3
[0036] Take 1kg of crystalline atorvastatin calcium, add 20L of ethanol, stir to dissolve, slowly add to 240L of isopropyl ether at room temperature, precipitate solid, continue to stir for 2 hours, vacuum filter, drain, and vacuum dry at 50°C for 12 hours to obtain 825 g of amorphous atorvastatin calcium, the X-ray powder diffraction pattern confirms the amorphous nature of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com