Phosphorescent chemical sensor for qualitatively detecting contrast of aminothiopropionic acid and homocysteine and use thereof
A photochemical sensor, homocysteine technology, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., to achieve the effect of eliminating interference and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of iridium complex: weigh IrCl 3 ·3H 2 O (5.52mmol) and the corresponding cyclometalated C^N ligand 4-(2-pyridine)-benzaldehyde (1.04mmol) were added to the double-necked flask, and vacuum-filled with nitrogen-vacuumized on the double-row tube , cycle three times, and finally protect the reaction system with nitrogen. After injecting a mixture of 2-ethoxyethanol and water (3:1, v / v) with a syringe, the reaction mixture was heated to 10°C, stirred for about 24 hours, and a precipitate formed. After the reaction was stopped, the reaction mixture was cooled to room temperature, and a precipitate was obtained by filtration. The resulting precipitate was washed with water and ethanol respectively to obtain a red solid iridium dichloro bridge compound (pba) 2 Ir(μ-Cl) 2 Ir(pba) 2 . Weigh iridium dichloride bridge compound (0.16mmol) and bipyridine ligand (0.32mmol) and join in the double-necked flask, vacuumize-fill nitrogen-vacuumize on the double-row tube,...
Embodiment 2
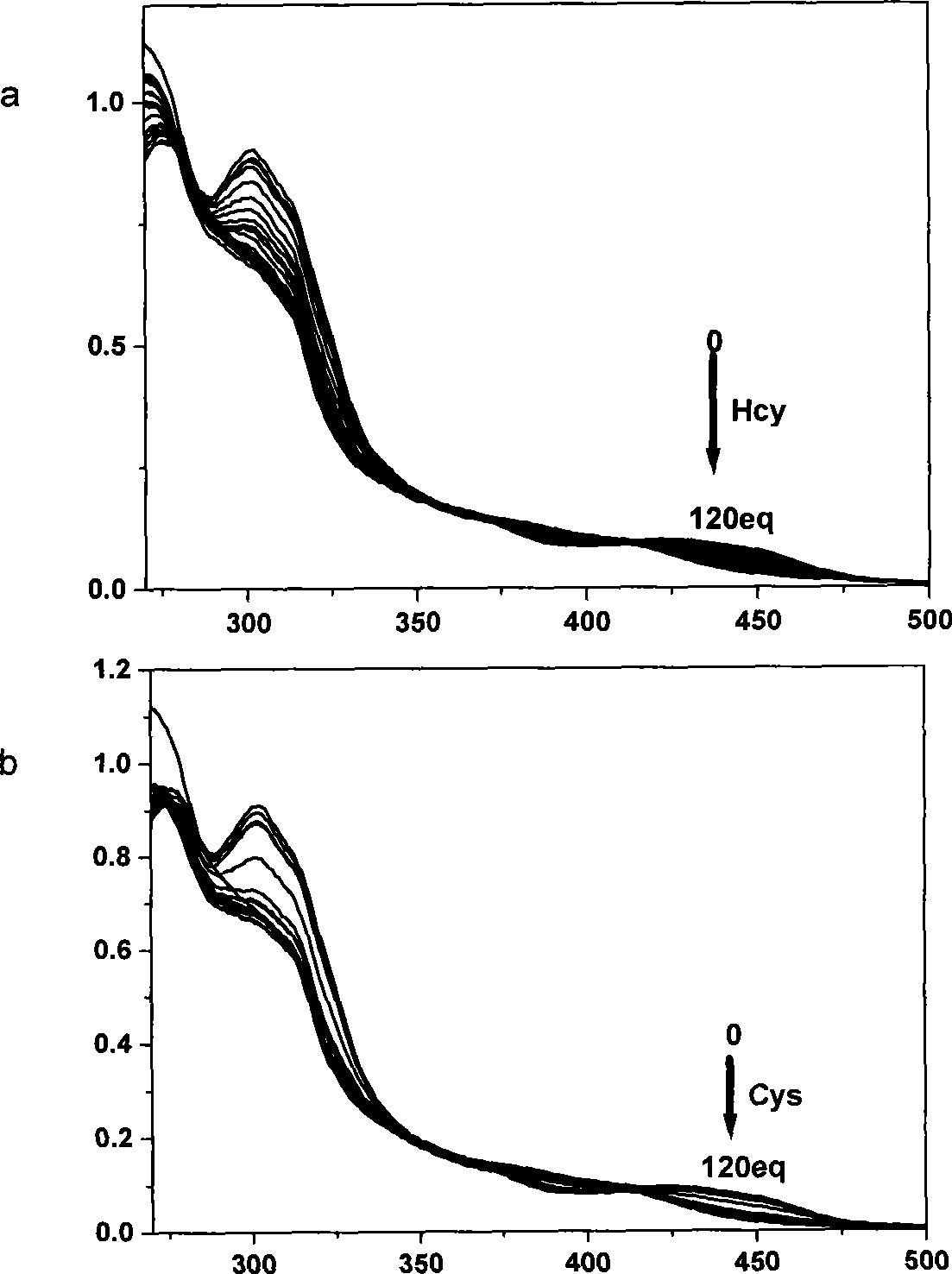
[0024] The ultraviolet spectrum test of the response of the complex to mercapto amino acids: the iridium complex was dissolved in the buffer solution of DMSO and HEPES, the pH value was 7.2, the volume ratio of the two was 9:1, the concentration of HEPES was 50mmol / L, and the The concentration is 20 μmol / L, and then add homocysteine and cysteine solutions prepared in an equal volume of 0-120eq HEPES buffer. After equilibration, measure the UV spectrum, see figure 1 .
Embodiment 3
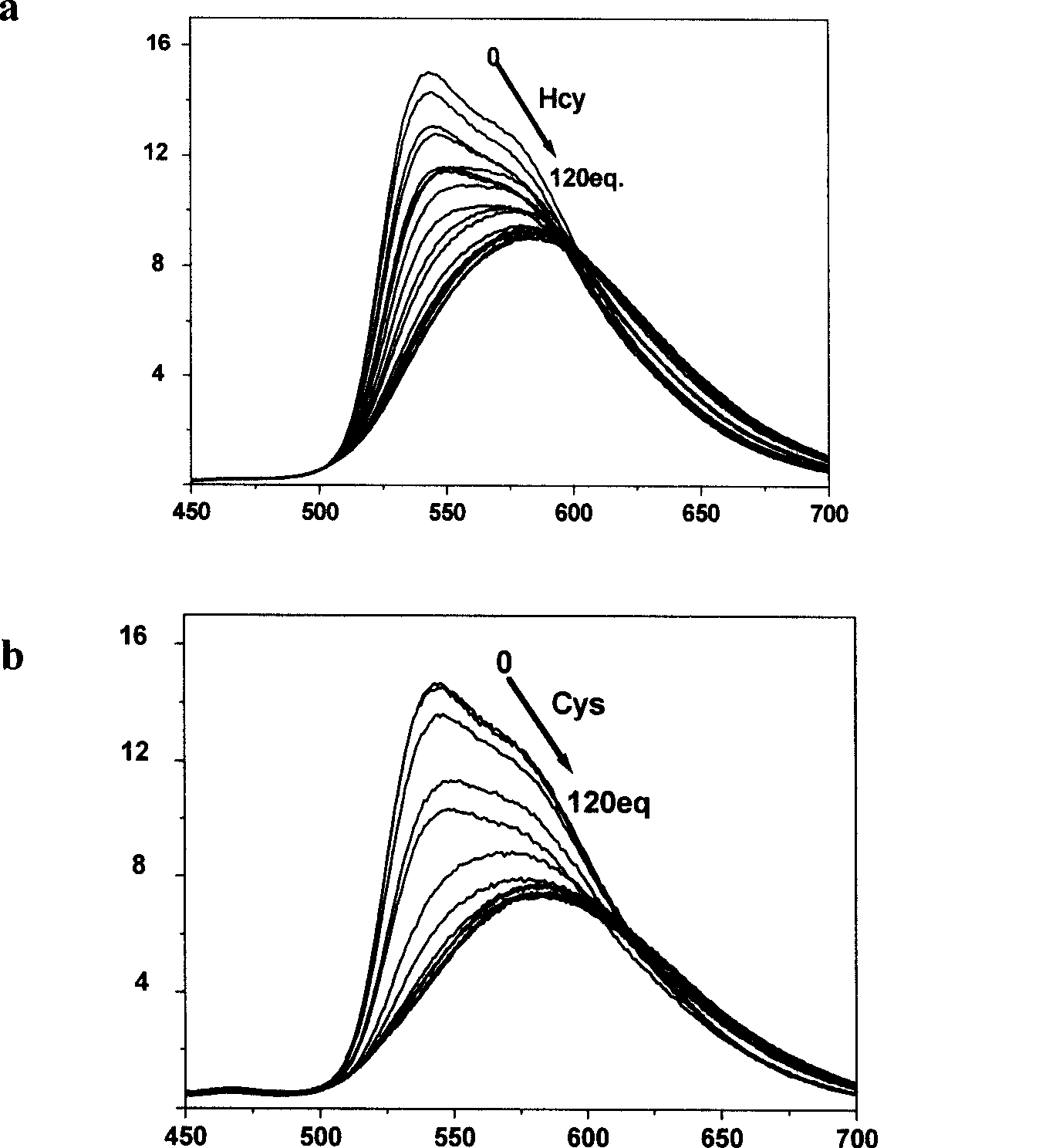
[0026] Phosphorescence spectroscopic test of the complex’s response to mercapto amino acids: the iridium complex was dissolved in a buffer solution of DMSO and HEPES, the pH value was 7.2, the volume ratio of the two was 9:1, and the concentration of HEPES was 50mmol / L. The concentration is 20 μmol / L, and then add homocysteine and cysteine solutions prepared in an equal volume of 0-120eq HEPES buffer. After equilibration, measure the phosphorescence spectrum, see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com