Methods for sulphation modification of 20(S)- ginsenoside Rh2, and separation and identification of sodium sulfovinate produced thereby
A technology of ginsenoside and sodium sulfate, which is applied in the fields of material separation, material analysis, organic chemistry, etc., can solve the problems that are difficult to modify and have not yet seen the sulfation modification method of saponins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
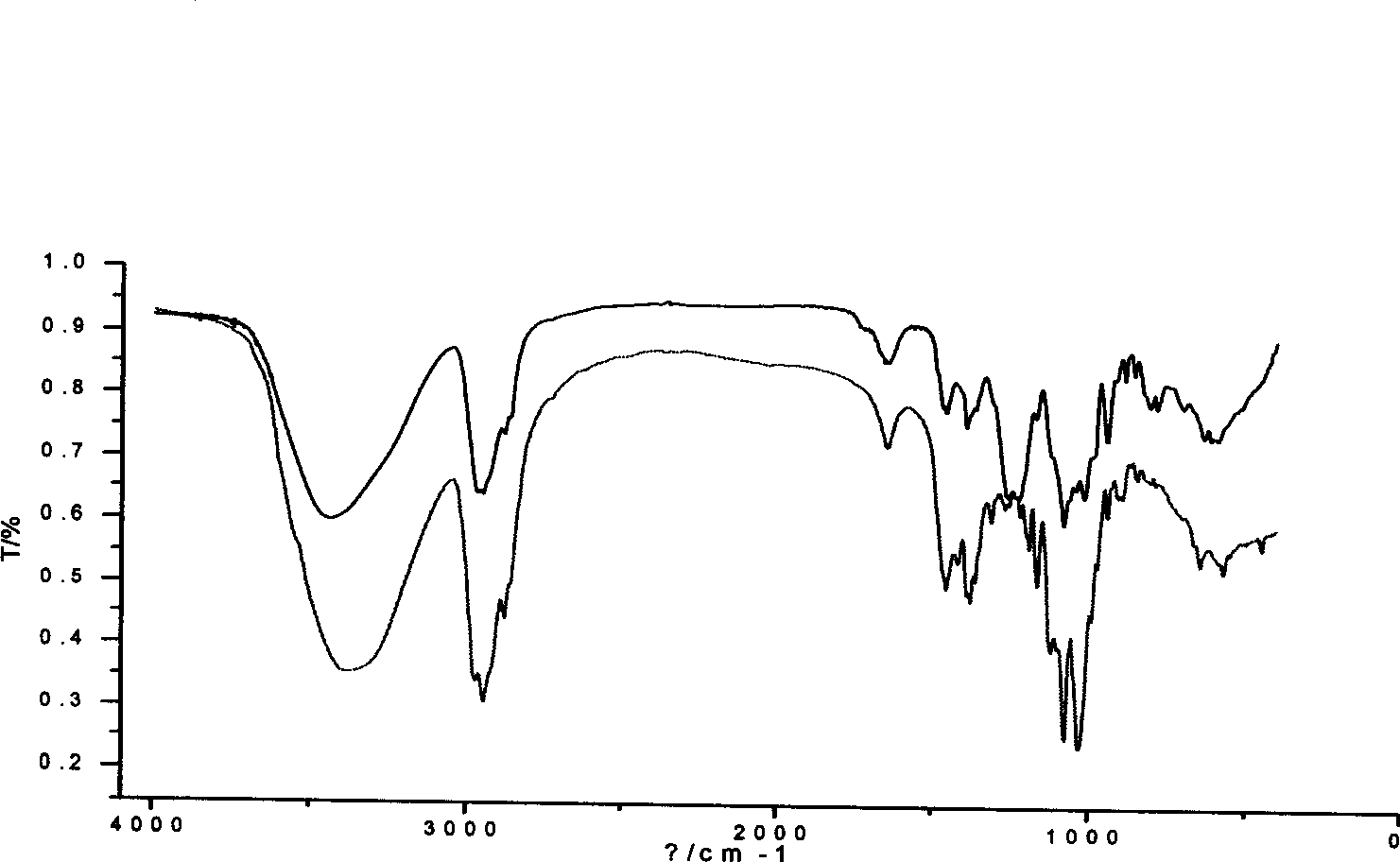
[0039] Add 2 mL of pre-cooled anhydrous pyridine into a flask equipped with a stirring device and a condensing device, place the flask in an ice-salt water bath, stir vigorously, and then add 84 μL of chlorosulfonic acid dropwise, and a pale yellow color appears Solid, end of preparation of esterifying agent. Accurately weigh 20(S)-ginsenoside Rh 2 200mg, dissolved in 2mL of anhydrous pyridine. Pour into the flask with the esterification reagent, place it at room temperature and stir continuously, and react for 3 hours.
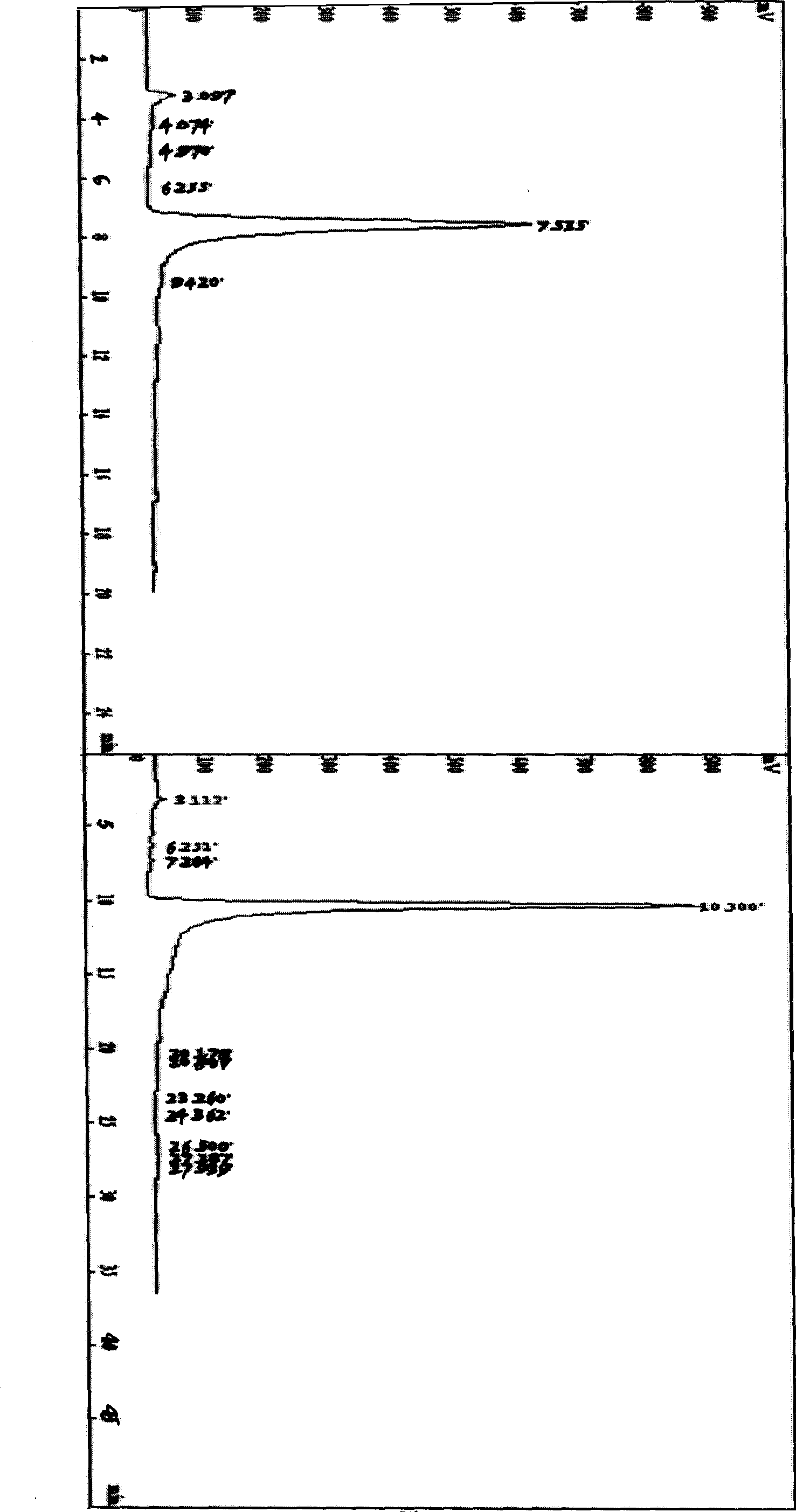
[0040] The reactant was neutralized to pH 7.0 with 1mol / L NaOH in an ice bath. A white precipitate was precipitated, filtered under reduced pressure, and the filtrate was dried to obtain 310 mg. With the expansion system (CHCl 3 —AcOEt—MeOH—H 2 O (15:40:22:10) to take the water phase) for TLC analysis, divided into I, II, III substances. Mix the sample with 2 times of silica gel, separate by silica gel column chromatography (silica gel 100-200 mesh, 1.5...
Embodiment 2
[0044] Add 2 mL of pre-cooled anhydrous pyridine into a flask equipped with a stirring device and a condensing device, place the flask in an ice-salt water bath, stir vigorously, and then add 21 μL of chlorosulfonic acid dropwise, and a pale yellow color appears Solid, end of preparation of esterifying agent. Accurately weigh 20(S)-ginsenoside Rh 2 100mg, dissolved in 2mL of anhydrous pyridine. Pour it into the flask with the esterification reagent, place it at room temperature and stir continuously, react for 3 hours, then heat to 30 and 40°C, and react for 3 hours each.
[0045] The reactant was neutralized to pH 7.5 with 1mol / L NaOH in an ice bath. A white precipitate was precipitated, filtered under reduced pressure, and the filtrate was dried to obtain 150 mg. With the expansion system (CHCl 3 —AcOEt—MeOH—H 2 O (15:40:22:10) to take the water phase) for TLC analysis, divided into I, II, III substances. Mix the sample with 2 times of silica gel, separate by silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com