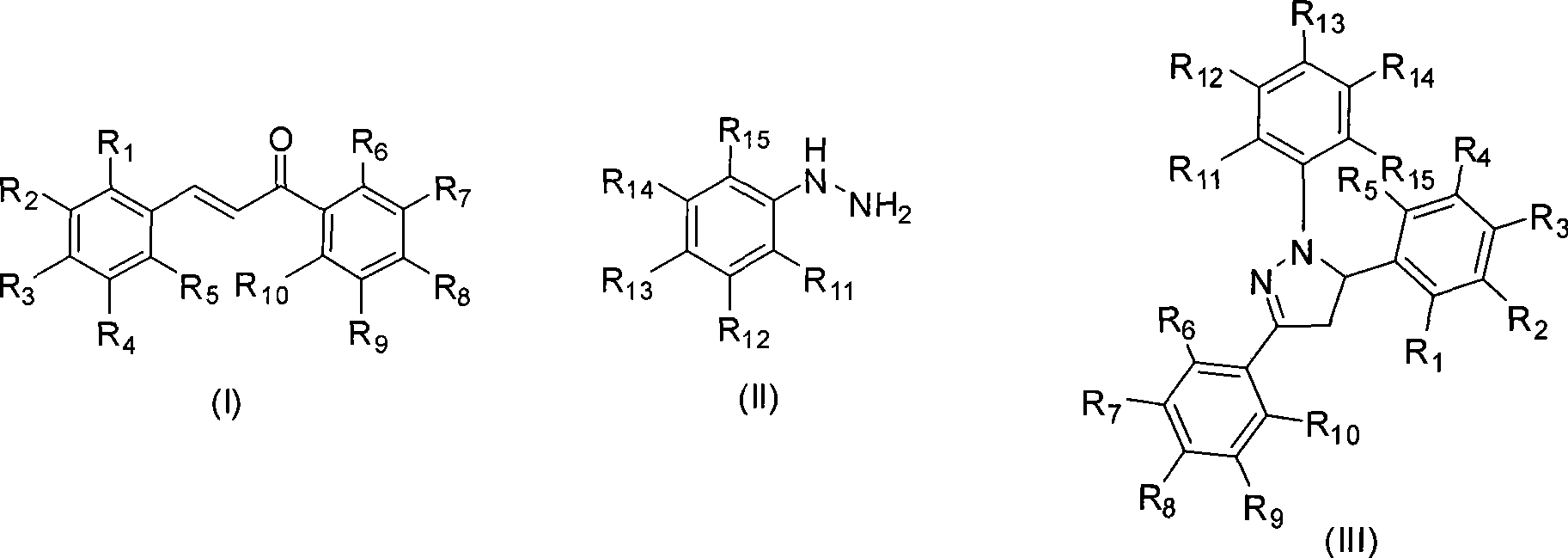
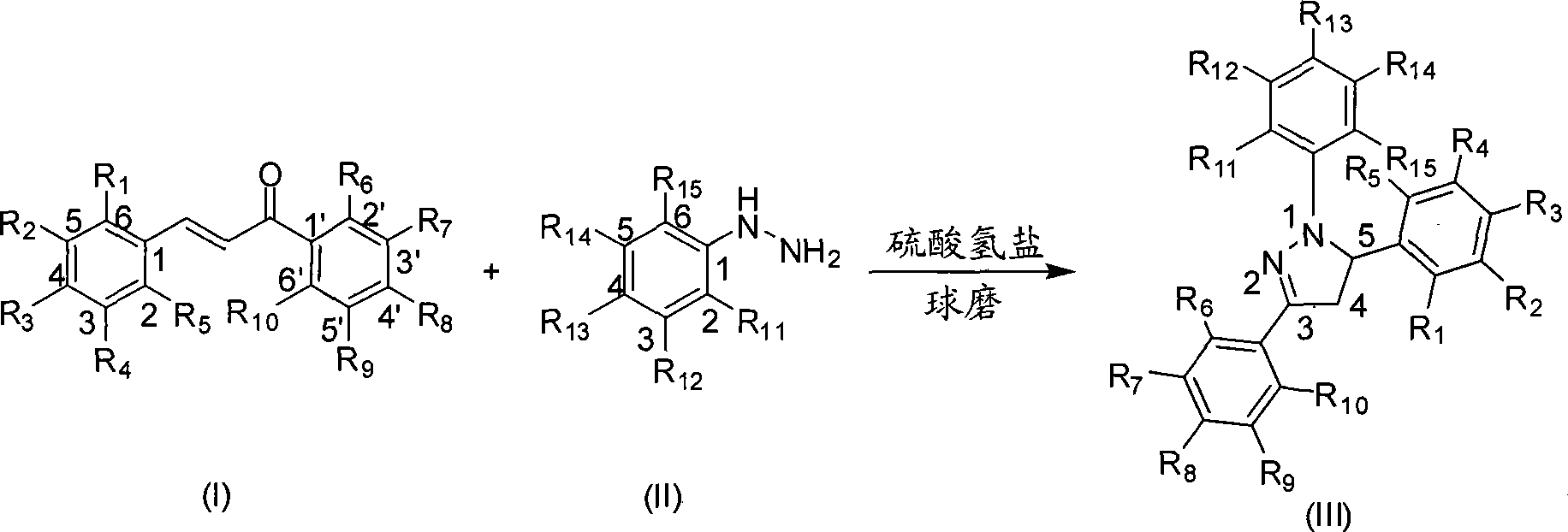
Mechanochemical preparation of 1,3,5-triaryl-2-pyrazoline compounds
A mechanochemical and compound technology, applied in 1 field, to achieve the effects of high reaction yield, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 1,3,5-triphenyl-2-pyrazoline
[0023] Add chalcone (2.08g, 10mmol), phenylhydrazine (1.08g, 10mmol), potassium hydrogen sulfate (0.14g, 1mmol) and silica gel (10g) into a 75mL ball milling jar, then add the milling ball and tighten the lid. Put the ball mill jar into the ball mill and run at 1290 rpm to make the mixture react for 5 min, TLC (sample dissolved in ethyl acetate) to track the progress of the reaction. After the reaction, the reaction mixture was washed with hot absolute ethanol, filtered, and the filtrate was boiled to evaporate part of the ethanol to saturation, cooled to room temperature, precipitated a solid, and dried to obtain 1,3,5-triphenyl- 2.72 g of 2-pyrazoline, the yield was 91%.
[0024] White solid, Mp: 134°C. IR (KBr): 3129, 1490, 1400cm -1 . 1 H NMR(400MHz, DMSO-d 6 ): δ(ppm)=7.76-6.71(m, 15H, ArH), 5.48(dd, J=5.2, 9.6Hz1H, ArCHN), 3.93(dd, J=9.6, 14.0Hz1H, CH 2 C=N), 3.11(dd, J=5.2, 14.0Hz1H, CH 2 C=N). 13 C NMR (100MHz...
Embodiment 2
[0025] Example 2: Preparation of 1,3,5-triphenyl-2-pyrazoline
[0026] Add chalcone (2.08g, 10mmol), phenylhydrazine (2.16g, 20mmol), potassium hydrogen sulfate (0.07g, 0.5mmol) and silica gel (10g) into a 75mL ball milling jar, then add the milling ball and tighten the lid. Put the ball mill jar into the ball mill and run at 1290 rpm to make the mixture react for 5 min, TLC (sample dissolved in ethyl acetate) to track the progress of the reaction. The separation and purification steps were the same as in Example 1, to obtain 2.77 g of 1,3,5-triphenyl-2-pyrazoline, with a yield of 93%, and the physical data were the same as in Example 1.
Embodiment 3
[0027] Example 3: Preparation of 1,3,5-triphenyl-2-pyrazoline
[0028] Add chalcone (2.08g, 10mmol), phenylhydrazine (5.41g, 50mmol), potassium hydrogen sulfate (0.27g, 2mmol) and silica gel (21g) into a 75mL ball milling jar, then add the milling ball and tighten the lid. Put the ball mill jar into the ball mill and run at 1290 rpm to make the mixture react for 5 min, TLC (sample dissolved in ethyl acetate) to track the progress of the reaction. The separation and purification steps were the same as in Example 1, to obtain 2.69 g of 1,3,5-triphenyl-2-pyrazoline, with a yield of 90%, and the physical data were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com