Improved method for the crystallization of intermediates of carbapenem antibiotics
一种结晶析出、结晶的技术,应用在氮杂环丁酮化合物领域,能够解决过滤时间延长、品质差、结晶稳定性差等问题,达到提高操作性和稳定性、控制结晶粒径分布、过滤性良好的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
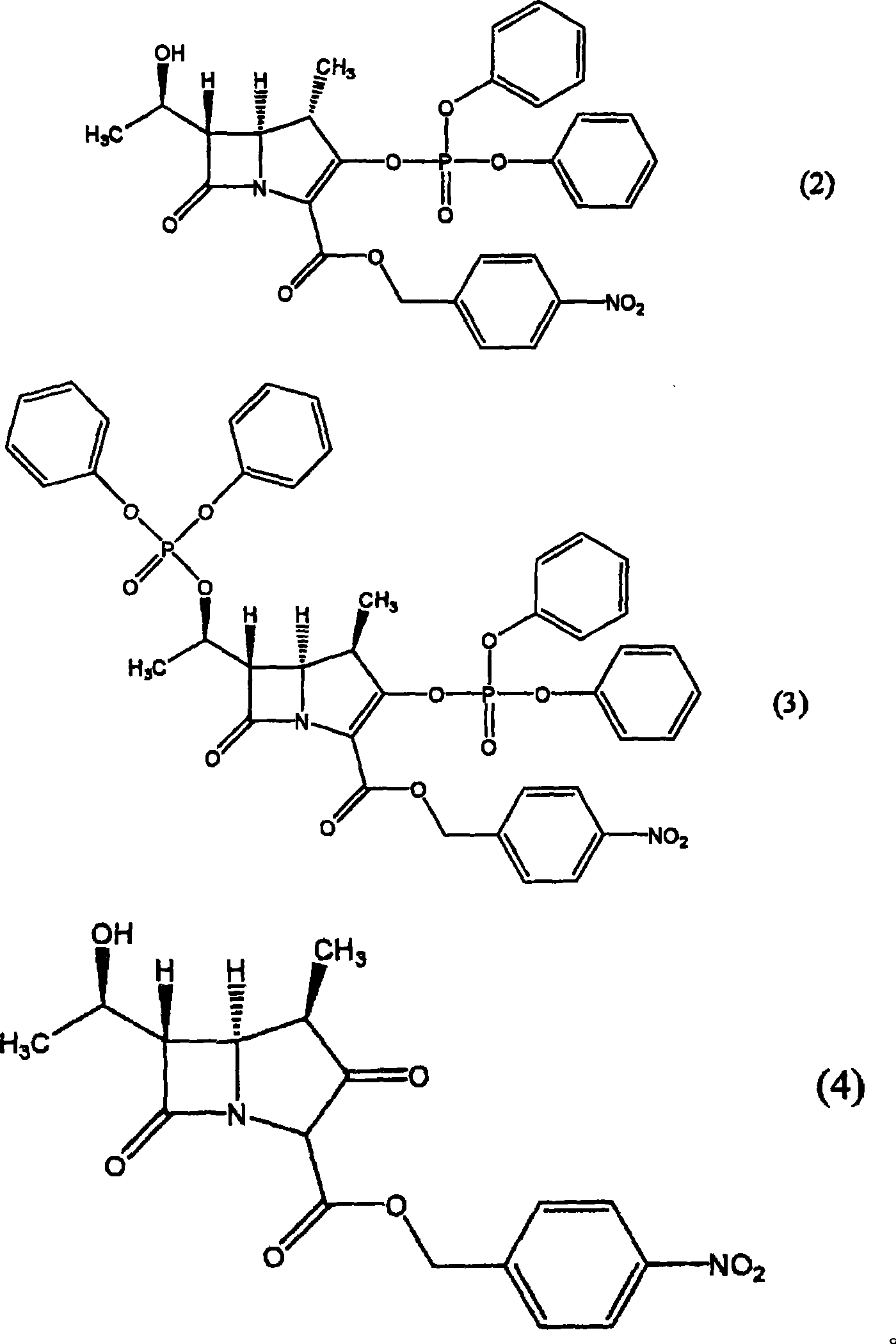
[0036] Regarding the preparation of seed crystals in the system of the present invention, it can be generated naturally, or can be carried out through concentration gradients, temperature gradients, external stimuli, and the like. As the preparation method in the system, it can be mentioned that by mixing the good solvent solution of the compound (1) and the hydrocarbon solvent, the method of precipitating the compound (1) can also be used. The method of precipitating the compound (1) from the hydrocarbon solvent may also be a method of precipitating the compound (1) by adding a good solvent solution of the compound (1) to the hydrocarbon solvent at one time, continuously or in portions. In addition, a method of precipitating the compound (1) may be performed by performing operations such as concentration, temperature rise, and cooling on a good solvent solution of the compound (1). In addition, these methods can also be combined.
[0037] When mixing with a hydrocarbon solve...
Embodiment
[0057] Hereinafter, the present invention will be further described by way of examples, comparative examples, and reference examples, but the present invention is not limited thereto.
reference example 1
[0059] Preparation of (4R,5R,6S)-1-aza-3-diphenoxyphosphoryloxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxobicyclo- [3.2.0] Good solvent solution of p-nitrobenzyl hept-2-ene-2-carboxylate (1)
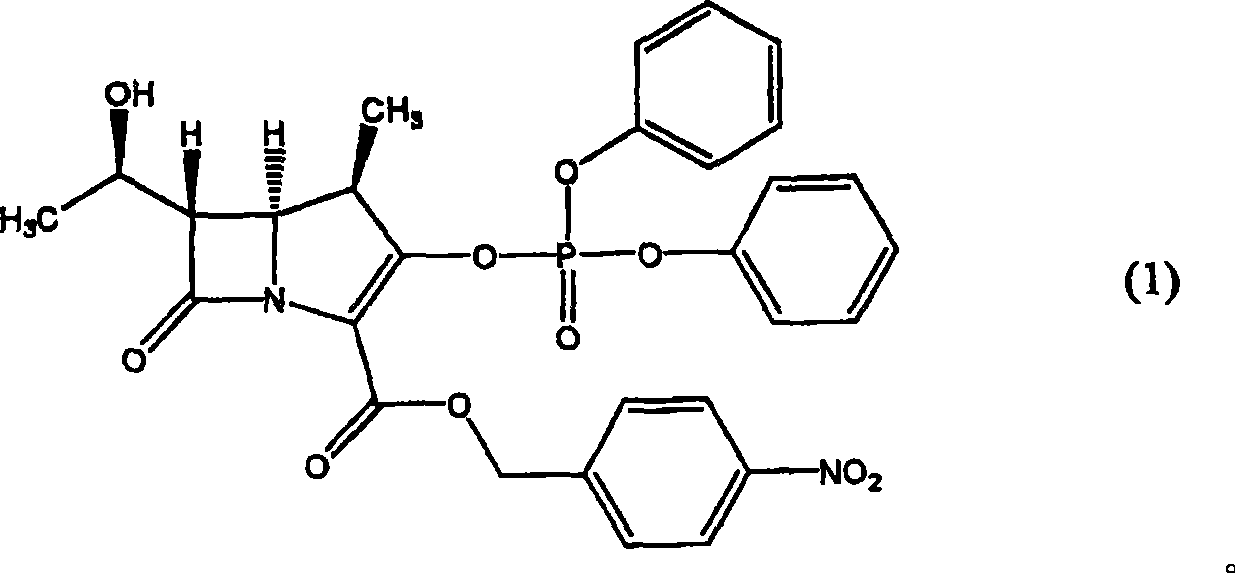
[0060]
[0061]Under nitrogen atmosphere, 21.6g (3S, 4R)-3-[(R)-1-hydroxyethyl]-4-[(R)-1-methyl-3-diazo-3- (p-Nitrobenzyloxycarbonyl)-2-oxopropyl]-2-azetidinone in 500mL of dichloromethane solution, add 20mL of dichloromethane solution dissolved with 135mg rhodium octanoate, and react at 40°C 6 hours. Then, the reaction liquid was cooled to -15° C., and 16.4 g of diphenyl chlorophosphate (diphenyl chlorophosphate) was added at the same temperature. Then, 9.5 g of N,N-diisopropylethylamine and 140 mg of N,N-dimethyl-4-aminopyridine were dissolved in 110 mL of dichloropyridine dropwise over 30 minutes at -15°C. Methane solution, reacted for 30 minutes. Subsequently, while keeping the reaction liquid below 10° C., 216 mL of 0.3 N aqueous hydrochloric acid solution and 216 mL of 5% sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com