Preparation of recombinant human hemoglobin with pichia stipitis
A technology for hemoglobin and Pichia pastoris, which is applied in the field of preparation of human hemoglobin, can solve the problems of different sequences, low expression efficiency of heterologous proteins, limited examples of industrial production, etc., and achieves the effect of less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
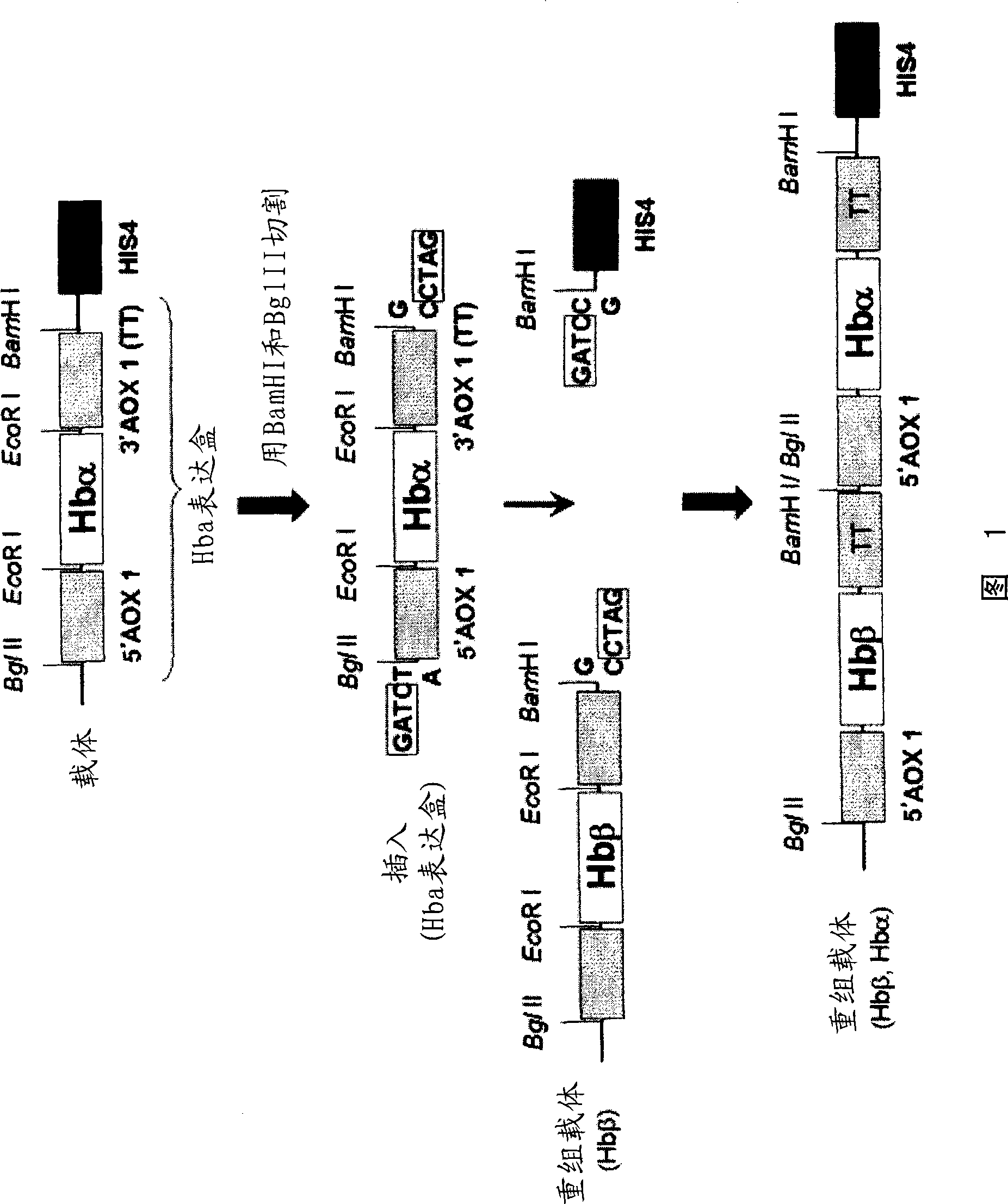
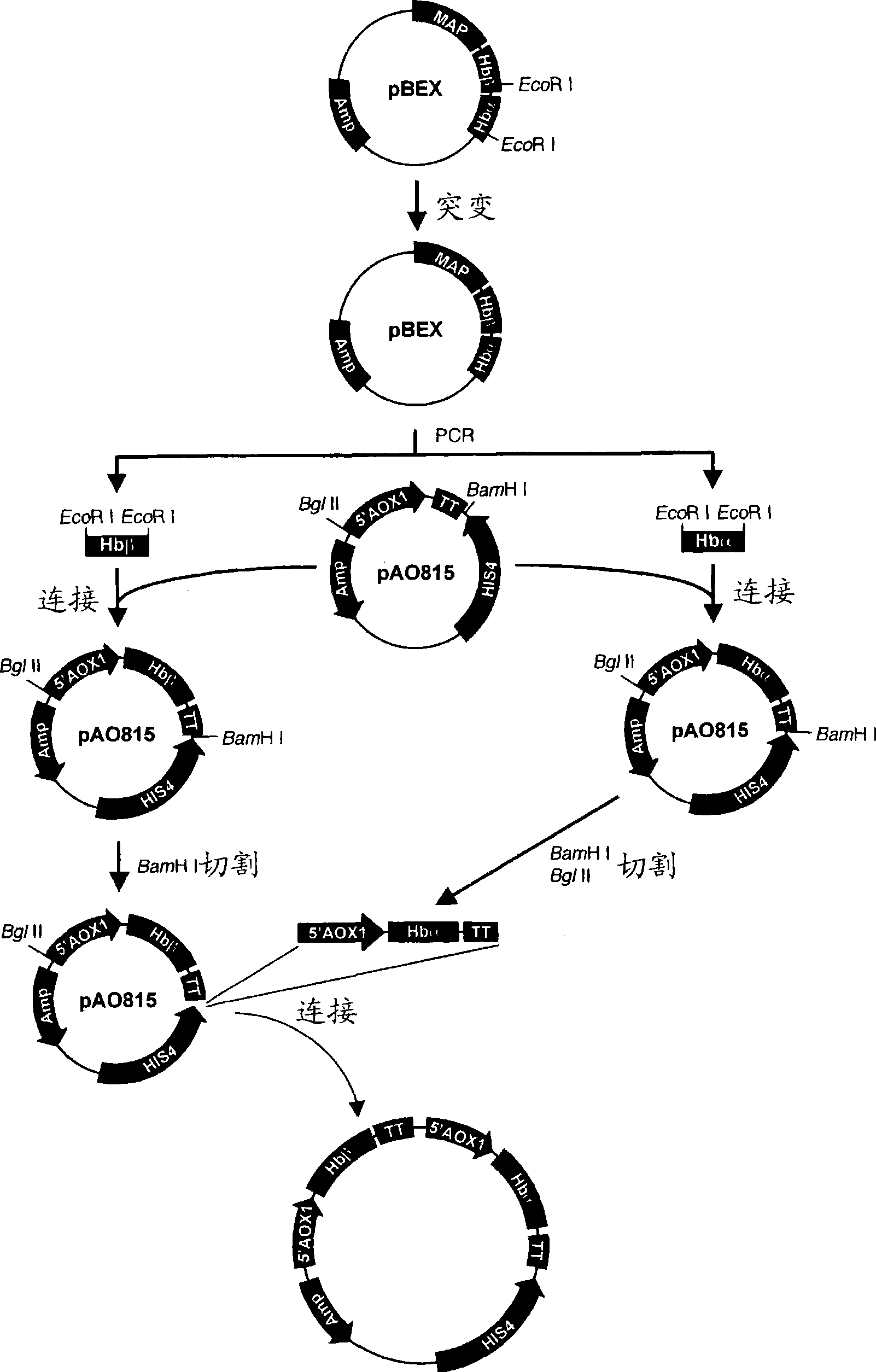
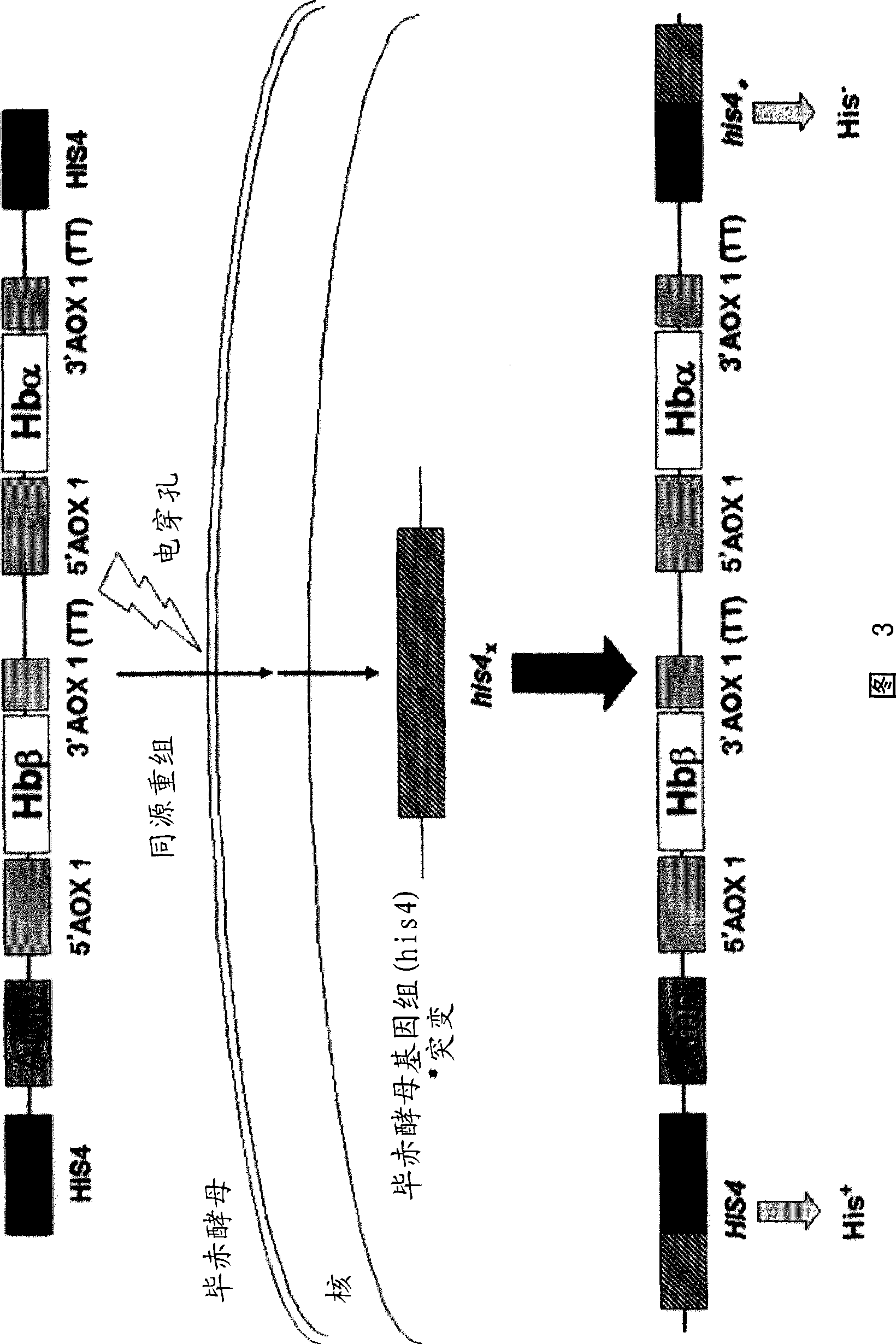
[0102] (1) Hemoglobin gene amplification
[0103] A plasmid in which the human hemoglobin gene was introduced into the plasmid pBEX (hereinafter referred to as "pBEX-Hb"; it can be obtained from the co-inventor of this patent, Koyuki Uno (Department of Pharmaceutical Research, Graduate School of Osaka University)) was used as a template, and the plasmids targeting hemoglobin were respectively used as templates. The Hbα-sense primer of SEQ ID NO: 5 and the Hbα-antisense primer of SEQ ID NO: 6 for the α chain DNA sequence, and the Hbβ-sense primer and the Hbβ-antisense primer of SEQ ID NO: 7 for the hemoglobin β chain DNA sequence As a synthetic primer, the EcoR I restriction enzyme site, which will hinder subsequent manipulation, was mutated (QuikChange XL Site-Directed Mutagenesis Kit, Stratagene). The reaction condition for mutation is that DNA is treated at 95°C for 30 seconds, followed by 12 cycles of denaturation (95°C, 30 seconds), annealing (55°C, 1 minute) and extension...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com