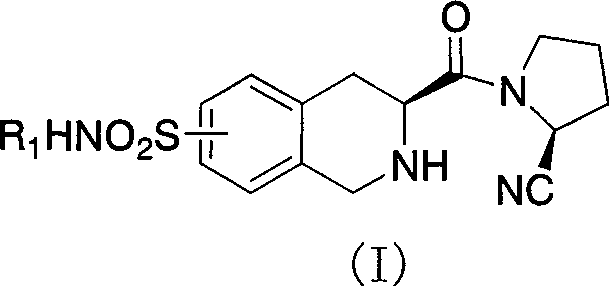
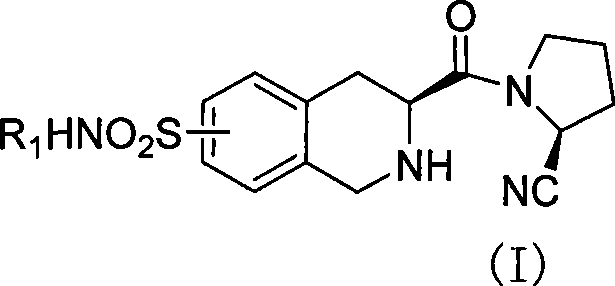
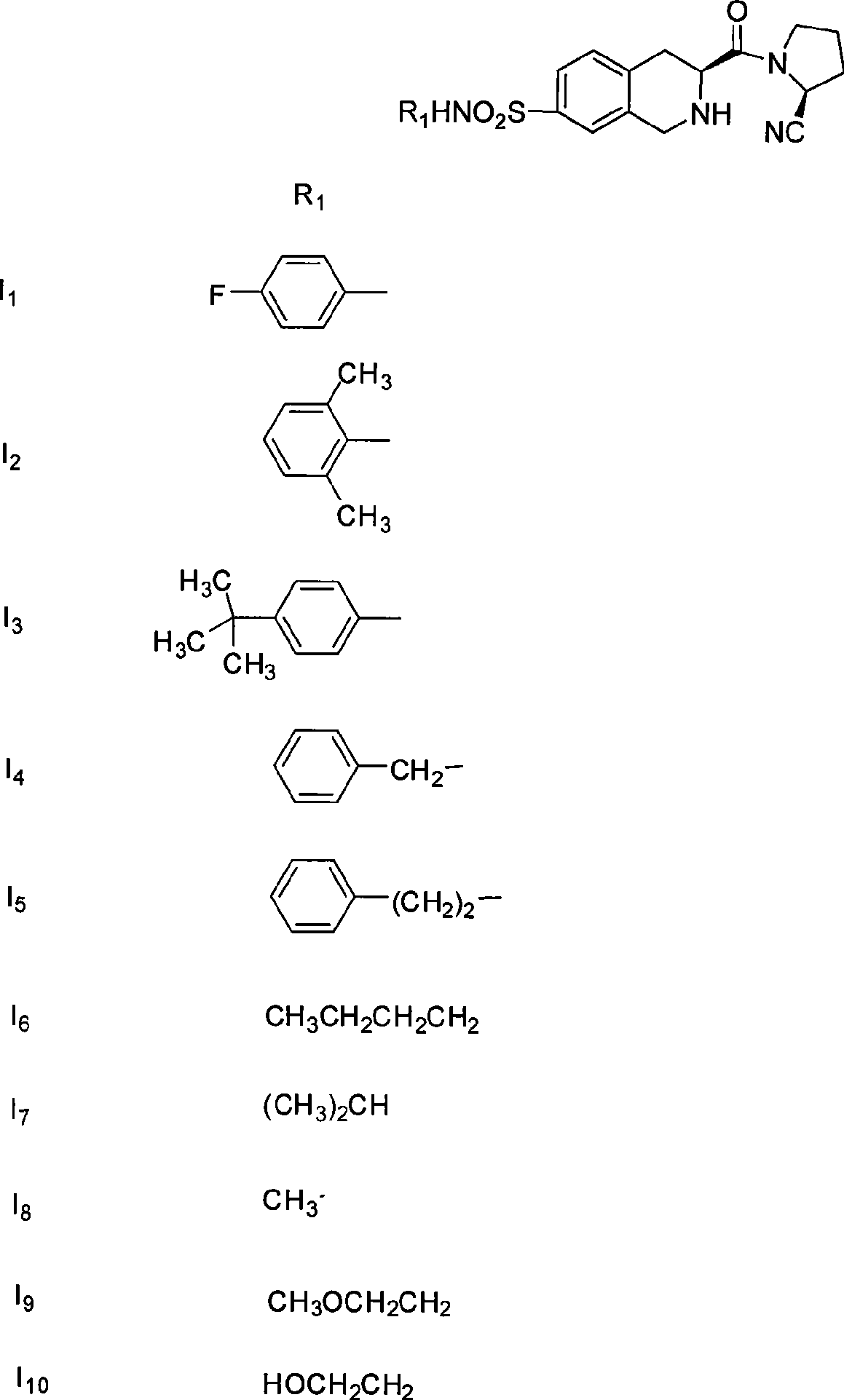
Uses of substituted tetrahydrochysene isoquinoline derivant
A use and compound technology, applied in the field of preparing antidiabetic drugs, can solve the problems of poor therapeutic effect on cardiovascular complications, hypoglycemic side effects, weak insulin sensitization effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]Preparation of (s)-2-acetyl-3-carboxy-1,2,3,4-tetrahydroisoquinoline (IX):
[0048] (s)-3-carboxy-1,2,3,4-tetrahydroisoquinoline 10g (0.047mol) mixed with 100ml acetone, 40ml sodium hydroxide aqueous solution (2mol / L), at room temperature, drop 8.01ml at the same time Acetyl chloride (0.094mol) and 2mol / L sodium hydroxide aqueous solution, after dropwise addition, keep the pH value of the reaction solution greater than 9, and continue to stir for 2 hours. Evaporate most of the solvent, acidify the remaining liquid with 3mol / L dilute hydrochloric acid, cool, and precipitate a white solid, filter, wash with water, and dry. The crude product weighs 9.1g (88.4%), and its melting point is 170-171°C (literature 171-173°C) .
Embodiment 2
[0050] Preparation of (s)-2-acetyl-3-formyl-1,2,3,4-tetrahydroisoquinoline (VIII):
[0051] At 0° C., 4.05 ml (0.057 mol) of thionyl chloride was added dropwise to 30 ml of anhydrous methanol. After the dropwise addition was completed, the mixture was stirred at room temperature for 2 hours. Add 5.0 g (0.023 mol) of (s)-2-acetyl-3-carboxy-1,2,3,4-tetrahydroisoquinoline to the above reaction solution, heat to reflux for 3 hours, and evaporate the solvent. Add ethyl acetate to dissolve, wash with saturated sodium bicarbonate and saturated brine respectively until neutral, dry over anhydrous sodium sulfate, evaporate the solvent, and dry to obtain a light yellow solid, the weight of the crude product is 5.04 g (94.1%).
Embodiment 3
[0053] Preparation of (s)-2-acetyl-3-carboxymethyl-7-chlorosulfonyl-1,2,3,4-tetrahydroisoquinoline (VII):
[0054] At -5°C, add 5.0 g (0.021 mol) of (s)-2-acetyl-3-methylcarboxylate-1,2,3,4-tetrahydroisoquinoline to 15 ml of chlorosulfonic acid in batches After the addition was complete, stir overnight at room temperature. The reaction solution was poured into a large amount of crushed ice, stirred, and filtered to obtain 12 g (wet weight) of a white solid, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com