Quantitative determination method for hepatitis b virus specificity cell toxicity T lymphocyte
A technology of hepatitis B virus and lymphocytes, which is applied in the field of immunoassay, can solve the problems that need to be further improved, achieve reliable results, reduce non-specific binding, and reduce the effect of background staining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
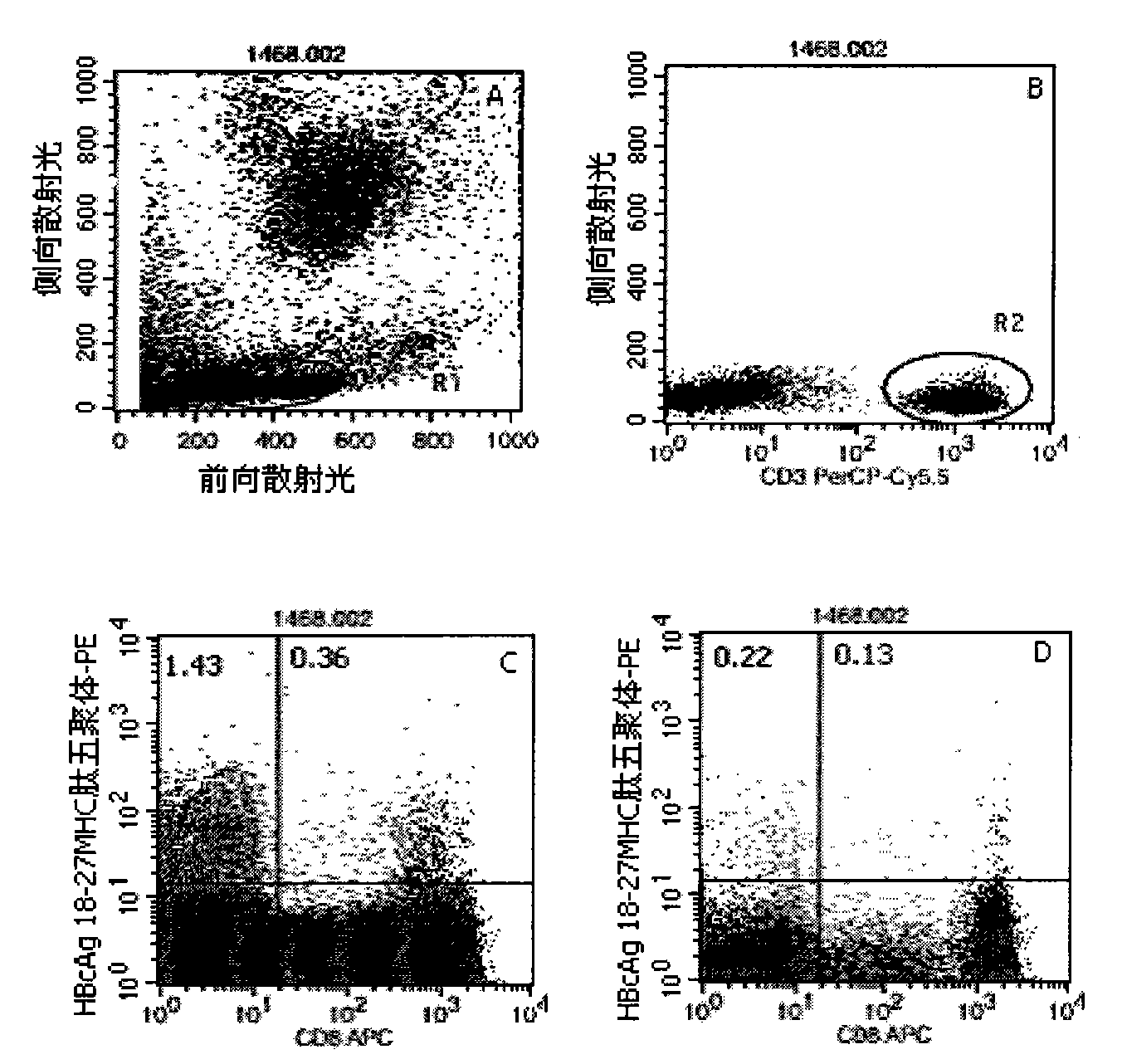
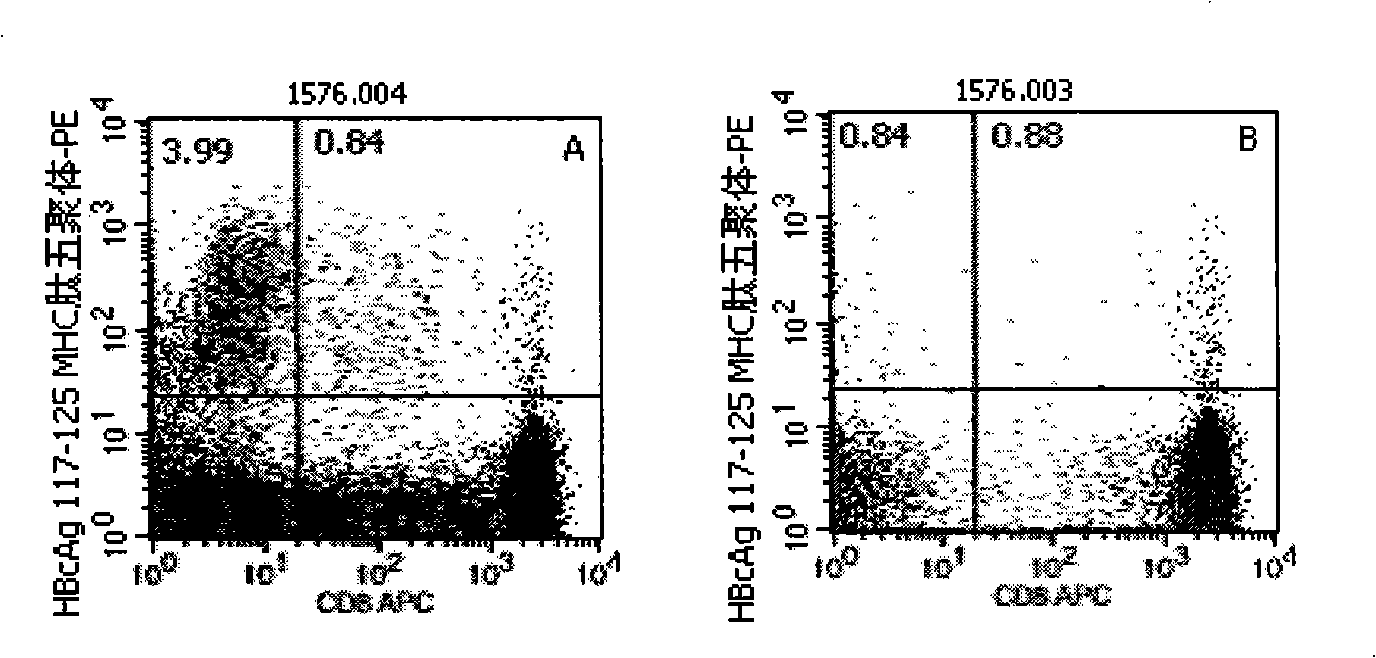
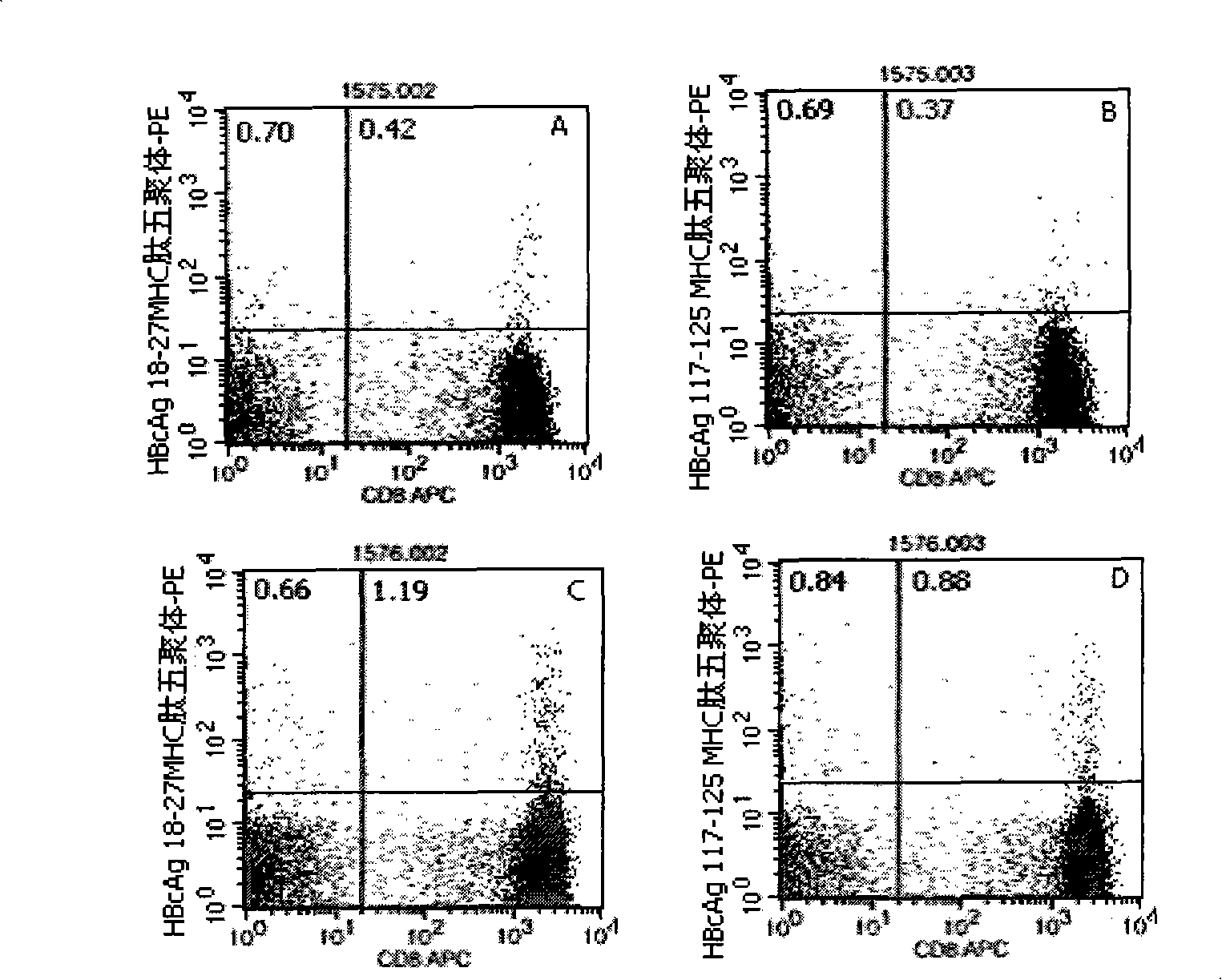
[0046] Embodiment 1: detection process of the present invention
[0047] 1. Marking of HLA-A2 and HLA-A24 gene subtypes: the purpose is to conduct preliminary screening of HLA-A2 and HLA-A24 gene subtypes in patients with hepatitis B, and the detection is carried out according to the instructions: the method is to add different Fluorescein thiocyanate (FITC)-labeled mouse anti-human immunoglobulin G1 (IgG1)- and phycoerythrin (PE)-labeled mouse anti-human IgG1 10 μl each, the reagents are all products of BD Company in the United States; in the detection tube Add 10 μl of mouse anti-human HLA-A2-FITC (the reagent is the product of BD Pharmingen, USA) and 10 μl of mouse anti-human HLA-A24-PE (the reagent is the product of Japan MBL Company) respectively, and incubate at 22-25°C in the dark 20-30min, with 3ml 0.01M pH 7.2 phosphate buffered saline (PBS) containing 0.1% bovine serum albumin (bovine serum albumin, BSA), centrifuge at 500g for 5min, discard the supernatant, then sha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com