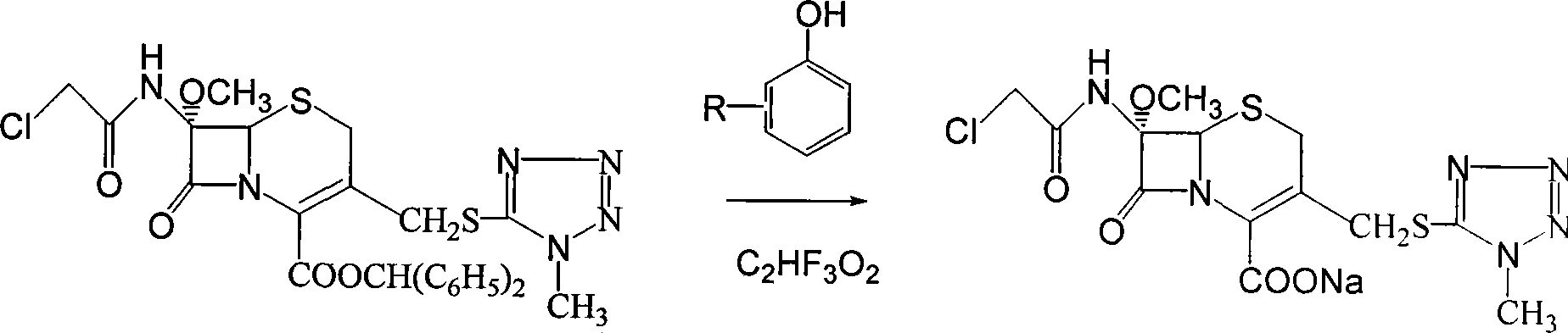
Method for preparing sodium 7-methoxy-7-chloracetylamino-3-methyltetrazole sulfidomethyl cephalosporanic acid
A technology for sodium methyl tetrazolium thiomethyl cephalosporanate and benzyl methyl tetrazolium thiomethyl cephalosporanate is applied in the field of pharmaceutical and chemical intermediates, and can solve the problems of difficult temperature control, unfavorable industrial production and complicated post-processing and other problems, to achieve the effect of easy control, simplified operation, and convenient industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] a. After adding 65Kg p-cresol solvent, 30Kg 7-methoxy-7-chloroacetamido-3-methyltetrazolium thiomethyl cephalosporanic acid benzyl ester and 4Kg trifluoroacetic acid in an absolutely dry and clean container, The reaction was carried out at 30°C under stirring, and the liquid phase in the reaction followed the reaction residue;
[0021] b. When the main reactant 7-methoxy-7-chloroacetamido-3-methyltetrazolium thiomethyl cephalosporanic acid benzyl ester remains less than 1%, add 100 liters of ethyl acetate to the reaction solution, Adjust the pH of the reaction solution to 7.0 to 8.0 with a sodium carbonate solution with a concentration of 2% by weight, then stir for about 15 minutes, and after standing, the reaction solution forms an organic phase and an aqueous phase;
[0022] c. Take the above water phase, add 180 liters of ethyl acetate to it, stir for half an hour until completely mixed, then adjust the pH to 1.0 to 2.0 with hydrochloric acid with a concentration of...
Embodiment 2
[0025] Embodiment 2: the difference between this embodiment and embodiment 1 is to replace 65Kg p-cresol solvent with 65Kg m-cresol.
Embodiment 3
[0026] Embodiment 3: the difference between this embodiment and embodiment 1 is to replace 65Kg p-cresol solvent with 65Kg o-cresol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com