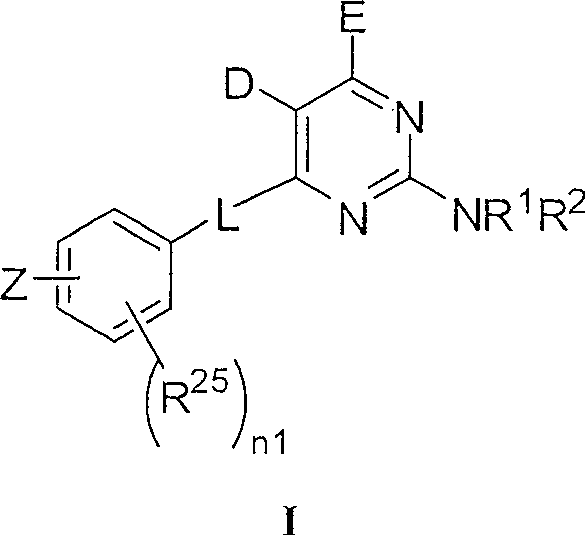
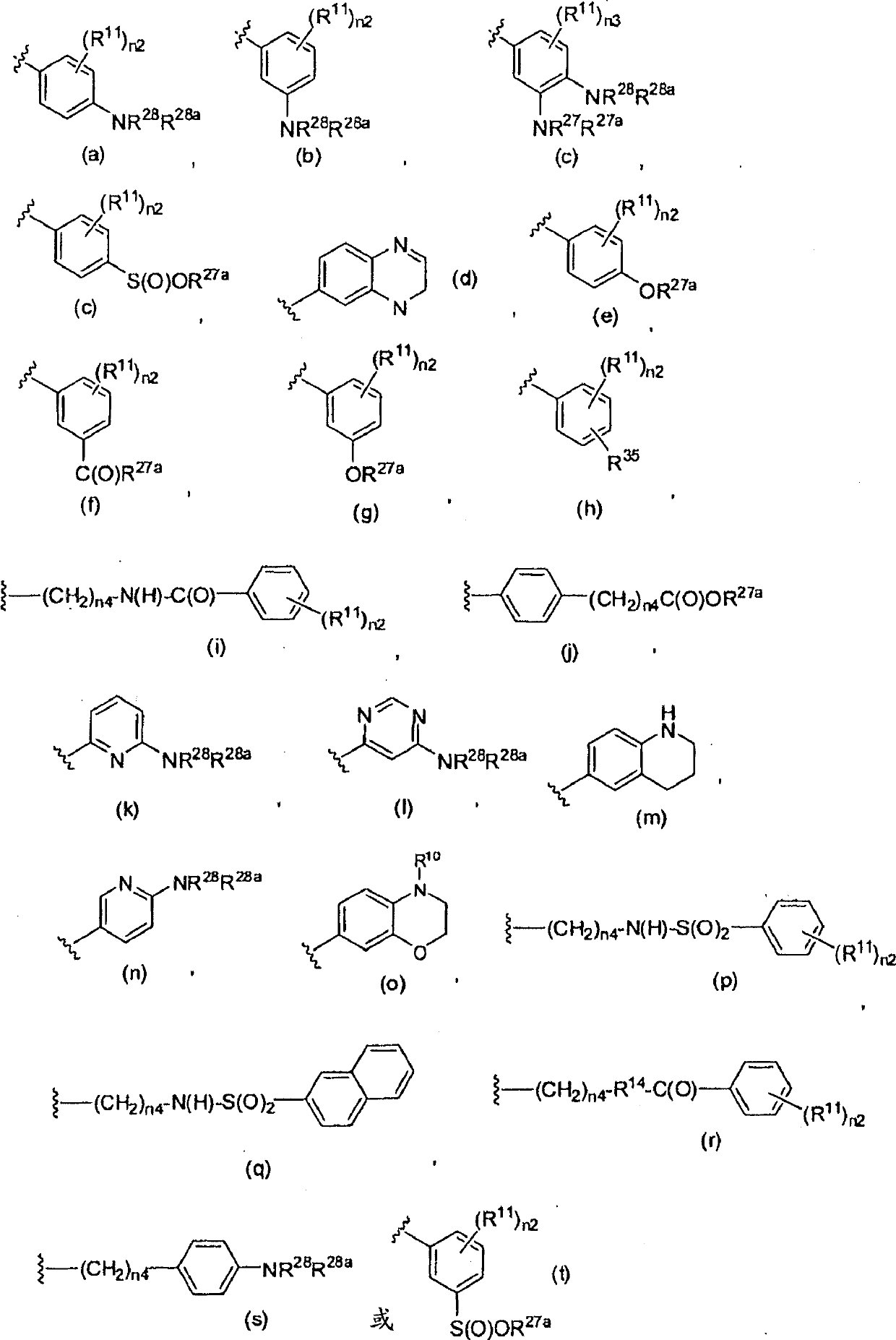
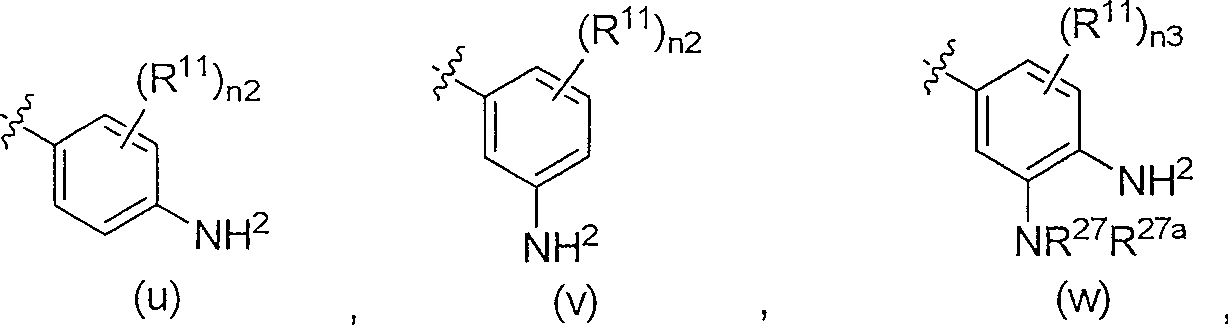
4-aryl-2-amino-pyrimidines or 4-aryl-2-aminoalkyl-pyrimidines as jak-2 modulators and pharmaceutical compositions containing them
A kind of technology of cycloalkylalkyl, heterocycloalkylalkyl, applied in 4-aryl-2-amino-pyrimidine or 4-aryl-2-aminoalkyl-pyrimidine as JAK-2 regulator and Field of pharmaceutical compositions containing them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0480] N-(4-{2-[(3-aminophenyl)amino]pyrimidin-4-yl}phenyl)acetamide (Compound 58)
[0481] a) N-(4-(2-chloropyrimidin-4-yl) phenyl) acetamide (C 1 )
[0482]
[0483] Add 2,4-dichloropyrimidine A to the flask 1 (650mg, 4.4mmol), 4-acetamidophenylboronic acid B 1 (820 mg, 4.6 mmol), dichloro[1,1'-bis(diphenylphosphino)ferrocenepalladium (480 mg, 0.56 mmol, 15 mol%) and triethylamine (1.5 mL, 11 mmol). Ethylene glycol dimethyl ether (30mL) was added into the flask, and the mixture was washed with N 2 Purge for 5 minutes. The reaction mixture was heated under N 2 Stir at 80°C under atmosphere for 12 hours, then add ether and filter the reaction mixture. Product C was isolated by removing the solvent with a rotary evaporator 1 , which was used without further purification. LCMS: m / z248(M+H) + .
[0484] b) N-(4-{2-[(3-aminophenyl)amino]pyrimidin-4-yl}phenyl)acetamide (58)
[0485]
[0486] will hold C 1 (500 mg, 2.0 mmol) and 3-tert-butoxycarbonyl-amino-aniline ...
Embodiment 2
[0488] N-[3-({4-[4-(acetylamino)phenyl]pyrimidin-2-yl}amino)phenyl]-2,6-dichlorobenzamide (Compound 7)
[0489]
[0490] A flask was charged with 58 (638 mg, 2.0 mmol), 2,6-dichlorobenzoyl chloride G (350 μL, 2.4 mmol), diisopropylethylamine (1.1 mL, 6 mmol) and THF (50 mL). The reaction mixture was stirred at 70°C for 6 hours. The crude mixture was concentrated on a rotary evaporator and the crude product was purified by HPLC using TFA / ACN as eluent. The title compound 7 was isolated by precipitation from ACN and washed with ether.
[0491] 1 H-NMR (400MHz, d 6 -DMSO): 10.718ppm (s, 1H), 10.269ppm (s, 1H), 9.678ppm (s, 1H), 8.507ppm (d, 1H), 8.419ppm (s, 1H), 8.215ppm (d, 2H ), 7.758ppm (d, 2H), 7.608ppm (d, 2H), 7.532ppm (t, 1H), 7.472ppm (d, 1H), 7.380ppm (d, 1H), 7.301ppm (t, 1H), 7.216ppm(d, 1H), 2.085ppm(s, 3H); MS(EI)C 25 h 19 Cl 2 N 5 o 2 : 492.2 (MH + ).
Embodiment 3
[0493] N-(4-{2-[(4-morpholin-4-ylphenyl)amino]pyrimidin-4-yl}phenyl)acetamide (18)
[0494]
[0495] Add C to the flask 1 (500 mg, 2.0 mmol), 4-morpholinoaniline H (540 mg, 3.0 mmol) and nBuOH (10 mL). The flask was immersed in a 180°C oil bath for 30 minutes. The mixture was cooled to room temperature and the black residue was dissolved in DMF and MeOH. The product was purified by HPLC using TFA / ACN as eluent. The TFA salt was removed by extraction with sodium hydroxide and ethyl acetate to afford the title compound 18.
[0496] 1 H-NMR (400MHz, d 6 -DMSO): 10.533ppm (s, 1H), 9.408ppm (s, 1H), 8.447ppm (d, 1H), 8.114ppm (d, 2H), 7.813ppm (d, 2H), 7.705ppm (d, 2H ), 7.288ppm (d, 1H), 6.982ppm (brs, 2H), 4.65ppm (brs, 4H), 3.072ppm (brs, 2H), 2.108ppm (s, 3H); MS (EI) C 22 h 23 N 5 o 2 : 390.3 (MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com