Anion receptor based on nitrofuran formyl hydrazone and phenolic hydroxyl and preparation and use of organagel thereof
A technology of nitrofuroylhydrazone and organogel, which is applied in organic chemistry, analyzing materials through chemical reactions, and analyzing materials through observing the impact on chemical indicators, etc., which can solve the inconvenience of carrying and storing solutions And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the synthesis of anion acceptor compound
[0041] (1) Synthesis of intermediate 2,6-diformyl-4-chlorophenol:
[0042] a. Preparation of 2,6-dimethylol-4-chlorophenol
[0043] Slowly heat the mixture of 15 grams of p-chlorophenol, 7.8 grams of paraformaldehyde and 1.2 grams of sodium hydroxide to 60 ° C, 4-chlorophenol is melted, paraformaldehyde is suspended in it, and the reaction is stirred at 60 ~ 65 ° C for 1 h , the reaction solution was khaki and then purple-red, and the reaction mixture was stirred at about 65°C for 3.5h, the reactant was agglomerated, and the reaction was terminated; the agglomerated reaction mixture was dissolved in 100mL of hot methanol, and the Adjust the pH to about 2-3; cool to room temperature, precipitate 2,6-dimethylol-4-chlorophenol, filter and collect the product. It was recrystallized with methanol to obtain light khaki crystals. Yield 65%.
[0044] b. Preparation of 2,6-diformyl-4-chlorophenol
[0045] Add 3.8 g (0...
Embodiment 2
[0050] Embodiment 2, the preparation of anion acceptor organogel
[0051] Add 0.015 g of the above-prepared anion acceptor compound into 1 mL of DMF, heat to 70-120° C. to dissolve, form a DMF solution with a mass percentage of 1.5%, and form a stable anionic organic gel after cooling to room temperature.
Embodiment 3
[0052] Embodiment 3, detect anion with anion acceptor organogel
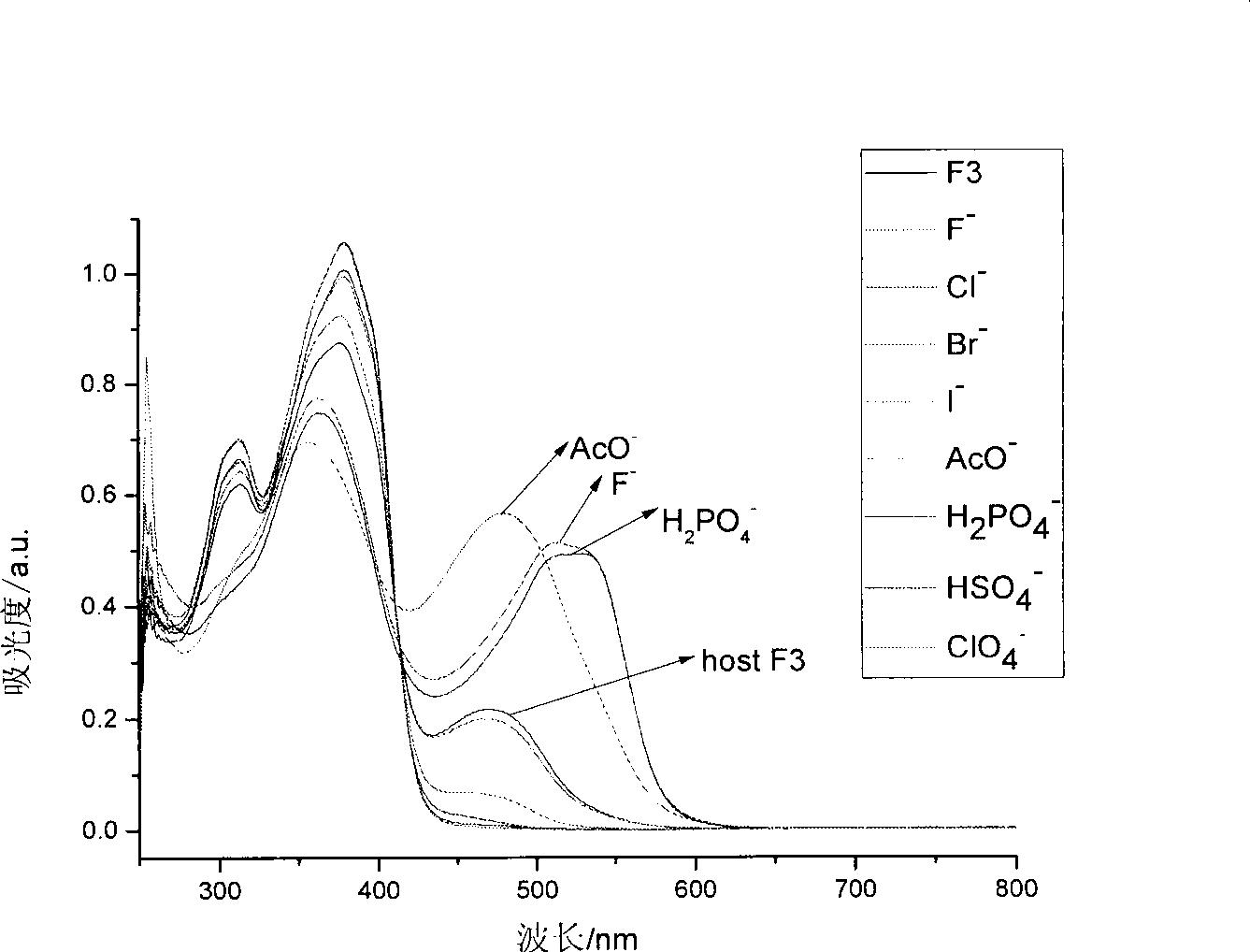
[0053] Take a small amount of anion acceptor organogels, place them on a white drip plate, and drop F on the organogels respectively. - , Cl - , Br - , I - , CH 3 COO - , HSO 4 - , H 2 PO 4 - , ClO 4- Anionic tetrabutylammonium salt in DMSO solution (0.01mol / L); organic gel changes from yellow to orange, then it is F-solution; organic gel changes from yellow to red, then it is CH 3 COO - solution; organogel changes from yellow to orange, then H 2 PO 4 - solution; if the organic gel does not change color, it is a solution of other ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com