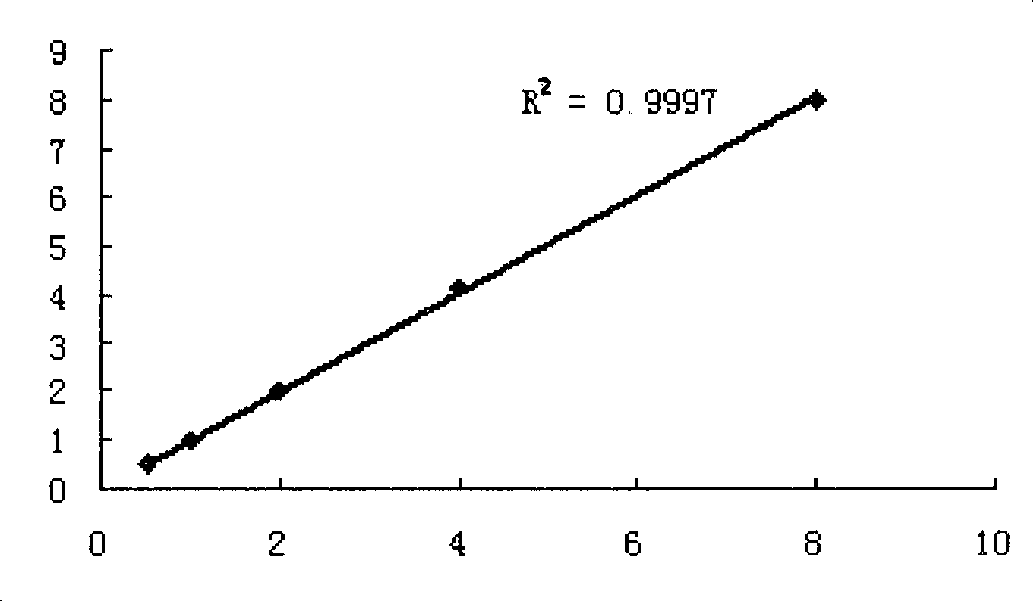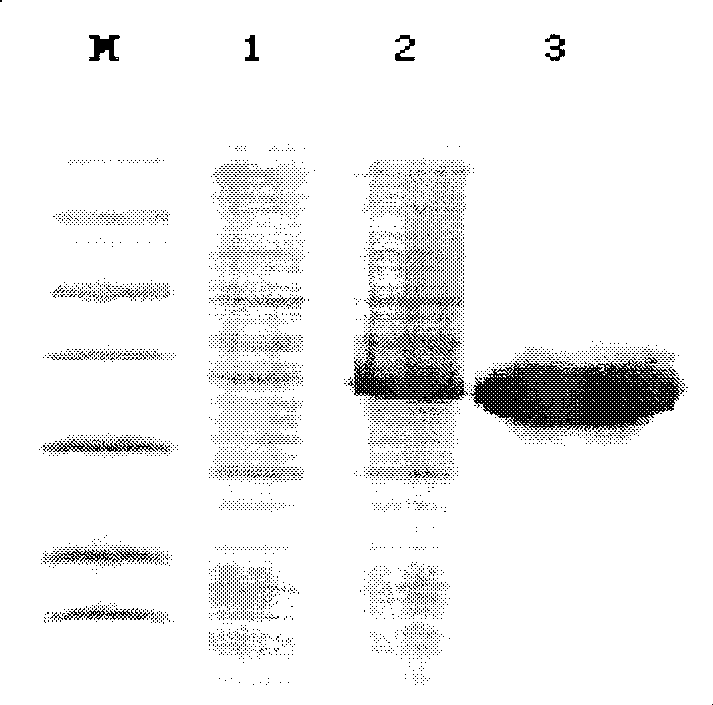Recombinant human cystatin C genes, and expression and use thereof
A technology of cystatin and C gene, which is applied in the field of recombinant human cystatin C gene and its expression and application, can solve the problems of poor purity, large batch-to-batch variation, low concentration of cystatin C protein, etc., to solve the bottleneck problem effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of recombinant soluble human cystatin C protein
[0024] (1) Clone the truncated cystatin C gene and construct the expression plasmid
[0025] Primers were designed according to the DNA sequence of the cystatin C gene, the gene was amplified by RT-PCR and ligated with the pMD18-T vector, transformed into Escherichia coli DH5a, single clones were identified by PCR to identify possible positive plasmids, and the plasmids were extracted and identified by double enzyme digestion. After DNA sequencing, the positive plasmid with the correct base and open reading frame was cut out with an appropriate enzyme and connected with the same double-digested PET32a(+) to construct a prokaryotic expression plasmid and transform the expression host strain Rosseta gami II. The positive host bacteria were identified by PCR. The plasmid vectors used in the above experiments were all kept in our laboratory. The expression strain Rosseta gami II was purchased from Merck Company,...
Embodiment 2
[0036] Preparation of Human Cystatin C Diagnostic Reagent
[0037] (1) Preparation of monoclonal antibodies against different epitopes
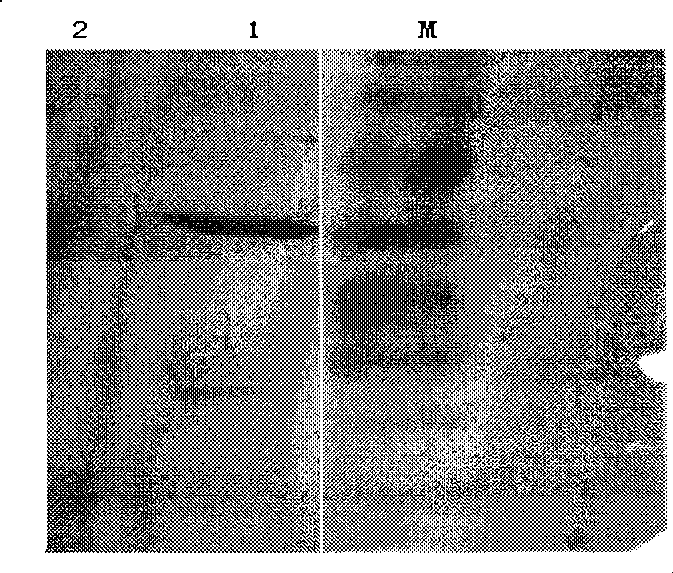
[0038] Purified recombinant cystatin C protein was prepared according to the monoclonal antibody preparation standard operating procedures to prepare multiple anti-clonal antibodies. A pair of monoclonal antibodies against different sites were screened by site stacking experiments. The specific steps are as follows: take the purified recombinant human cystatin C protein and dilute it to 10ug / ml and pack several plates according to the Elisa standard operating procedures (1-N, N is the number of monoclonal strains to be tested), seal and wash the plates and then add the first strain The monoclonal antibody reaches the saturation concentration of the antigenic site corresponding to the monoclonal antibody (the monoclonal antibody concentration required to saturate the site is completed through the pre-experiment), and incubate at 37°C. In the...
Embodiment 3
[0044] Preparation of Human Cystatin C Diagnostic Quality Control
[0045] Protect buffer (0.02M carbonate buffer, 20% glycerol, 0.8% sucrose, 0.5% BSA, 0.2‰ NaN 3 ) was diluted into five concentrations of 8ug / ml, 4ug / ml, 2ug / ml, 1ug / ml, and 0.5ug / ml, and then freeze-dried to make a human cystatin C diagnostic quality control product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com