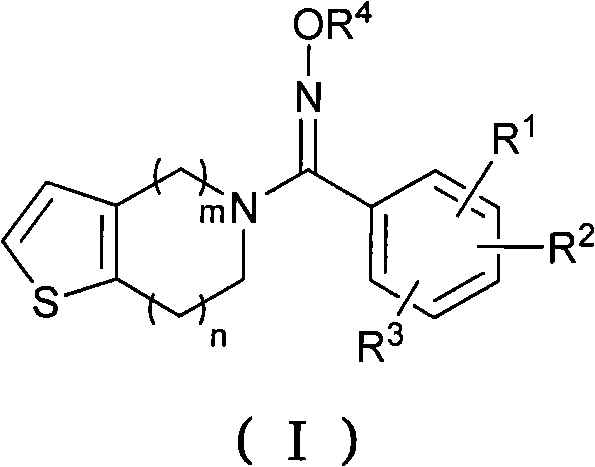
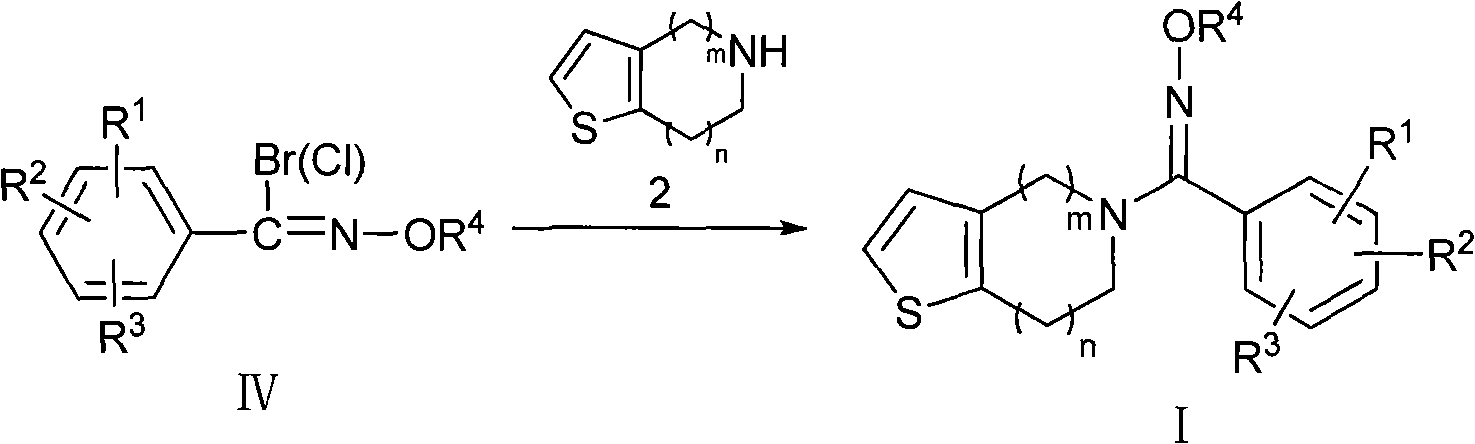
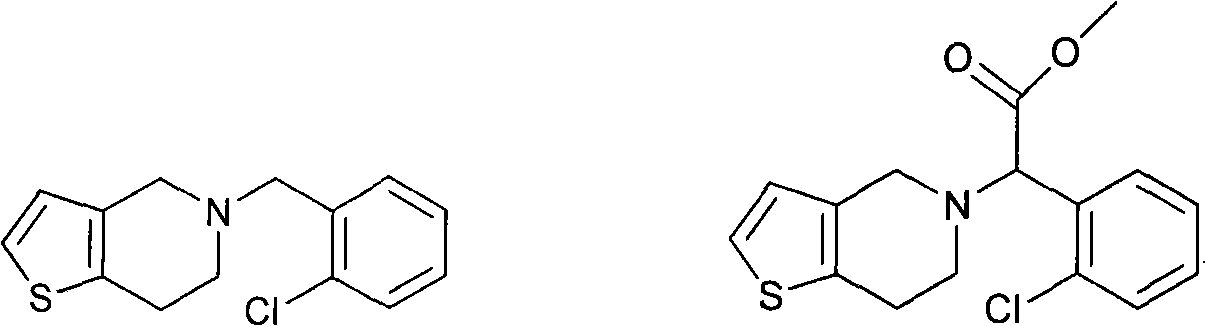
Oxime derivatives containing thienopyridine, preparation method and application thereof
A technology of pyridine and tetrahydrothiophene, which is applied in the field of medicine and can solve problems such as weak alkalinity, difficult purification, and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0087] Intermediate III-2
[0088]
[0089]Add 16.4g of 3-carboxy-5-methylbenzaldehyde into a reaction flask equipped with stirring, a condenser, and a thermometer, dissolve it with 45mL of anhydrous methanol, and add 11.2g of potassium hydroxide while stirring. Add 8.4 g of hydroxylammonium oxymethyl hydrochloride in batches to the reaction system. After the addition was complete, the reaction was continued at 35°C for 6.5h (the plate showed that the reaction was complete). Evaporate anhydrous methanol to dryness, wash the reaction liquid with 3×50mL water, extract with chloroform, fully dry over anhydrous sodium sulfate, filter, and evaporate chloroform under reduced pressure to obtain an off-white solid (HPLC: 98.3%). Rf = 0.32 [single site, developing solvent: v (dichloromethane): v (methanol) = 6: 1].
Embodiment 3
[0091] Intermediate III-3
[0092]
[0093] Add 14.8g of 2-methyl-4-ethylbenzaldehyde into a reaction flask equipped with stirring, a condenser and a thermometer, dissolve it with 40mL of anhydrous methanol, and add 9.0g of sodium hydroxide while stirring. Add 9.8 g of oxyethyl hydroxylammonium hydrochloride to the reaction system in batches. After the addition was complete, the reaction was continued at 55°C for 6h (the plate showed that the reaction was complete). Evaporate anhydrous methanol to dryness, wash the reaction solution with 3×50mL water, extract with dichloromethane, fully dry over anhydrous sodium sulfate, filter, and evaporate dichloromethane under reduced pressure to obtain a white solid (HPLC: 99.2%). Rf = 0.40 [single site, developing solvent: v (dichloromethane): v (methanol) = 6: 1].
[0094] Reference Example 4:
[0095] Intermediate III-4
[0096]
[0097] Add 12.4g of 2-fluorobenzaldehyde into a reaction flask equipped with stirring, a conde...
Embodiment 7
[0107] Intermediate III-7
[0108]
[0109] Add 15.9 g of 2-fluoro-3-chlorobenzaldehyde into a reaction flask equipped with stirring, a condenser, and a thermometer, dissolve it with 20 mL of acetone, and add 15.8 g of pyridine while stirring. Add 6.9 g of hydroxylammonium hydrochloride to the reaction system in batches. After the addition was completed, the reaction was continued for 6.5 h under reflux (the plate showed that the reaction was complete). The acetone was evaporated to dryness, the reaction solution was washed with 3×20 mL of water, extracted with chloroform, fully dried over anhydrous sodium sulfate, filtered, and the chloroform was evaporated under reduced pressure to obtain a white solid (HPLC: 99.8%). Rf = 0.32 [single site, developing solvent: v (dichloromethane): v (methanol) = 6: 1].
[0110] Reference Example 8:
[0111] Intermediate III-8
[0112]
[0113] Add 21.9g of 2-chloro-4-bromobenzaldehyde into the reaction flask equipped with stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com