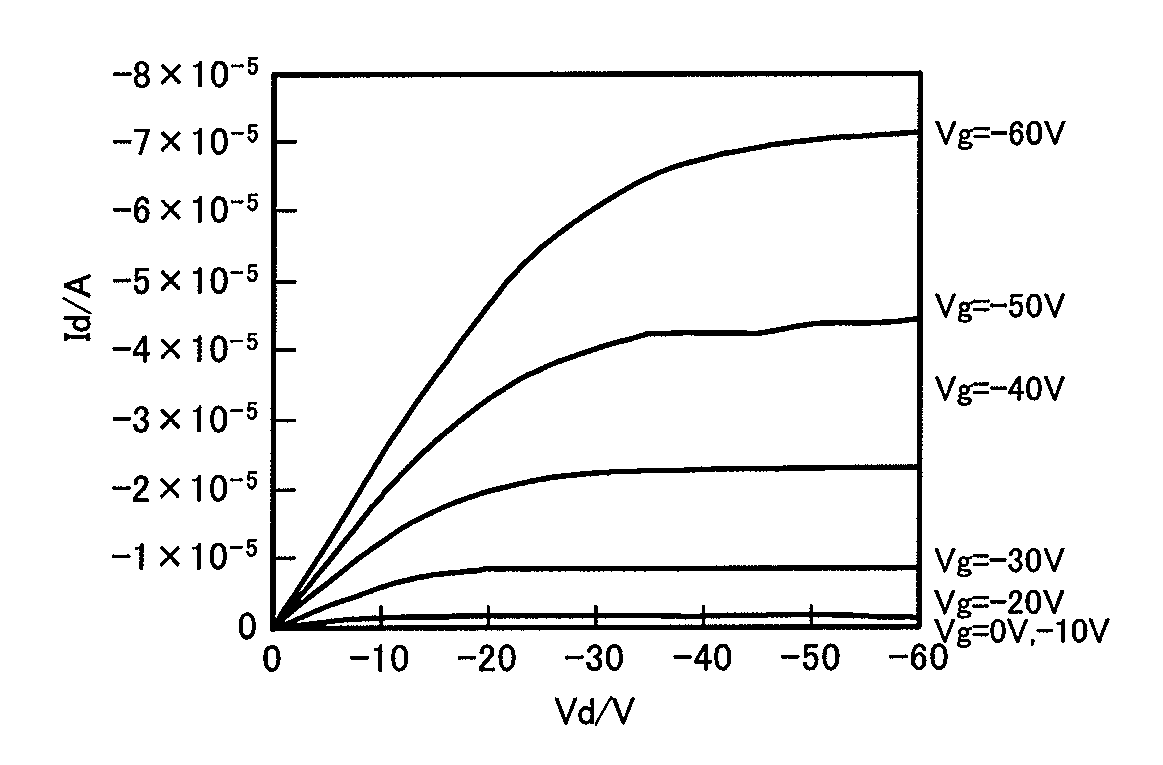
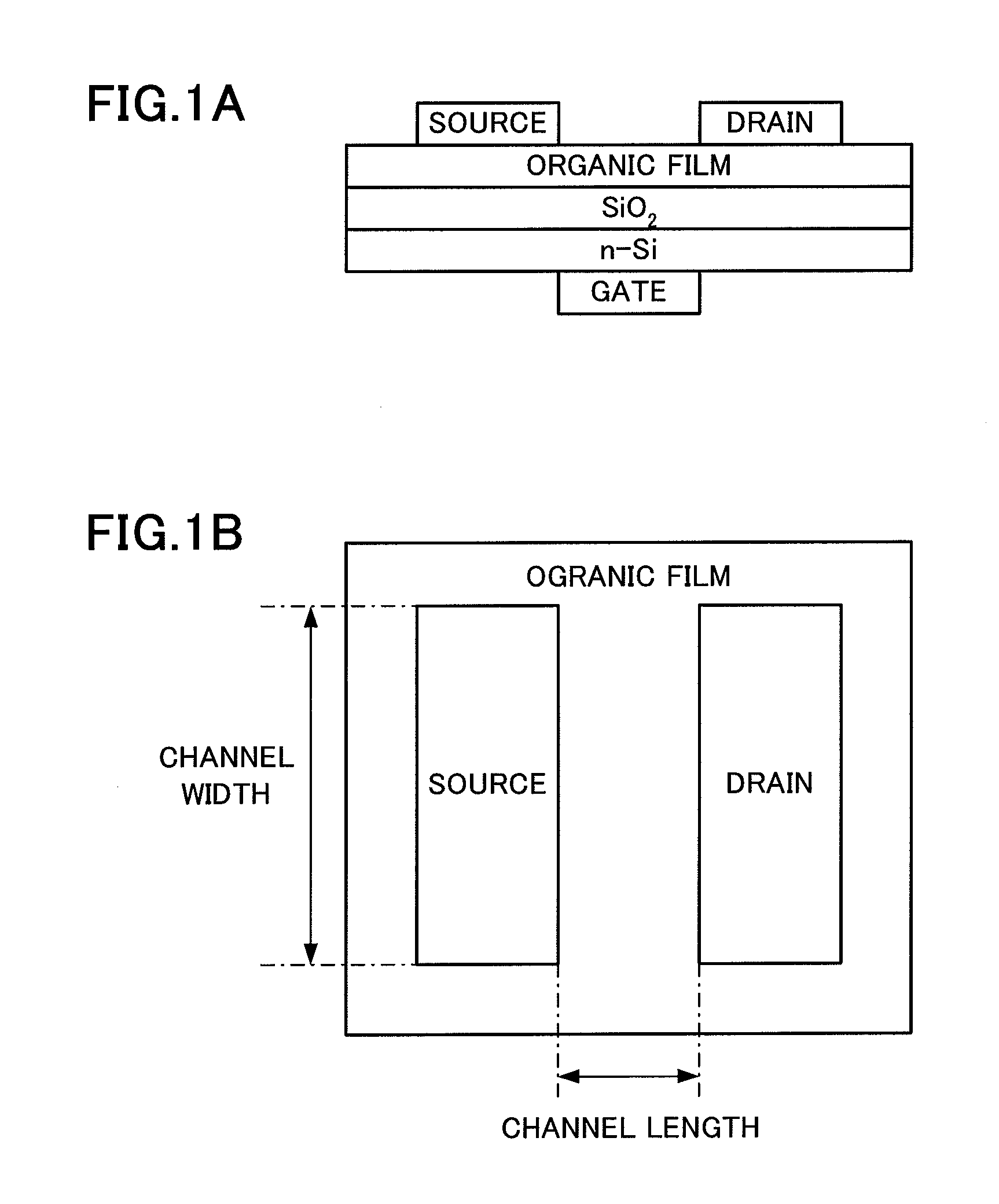
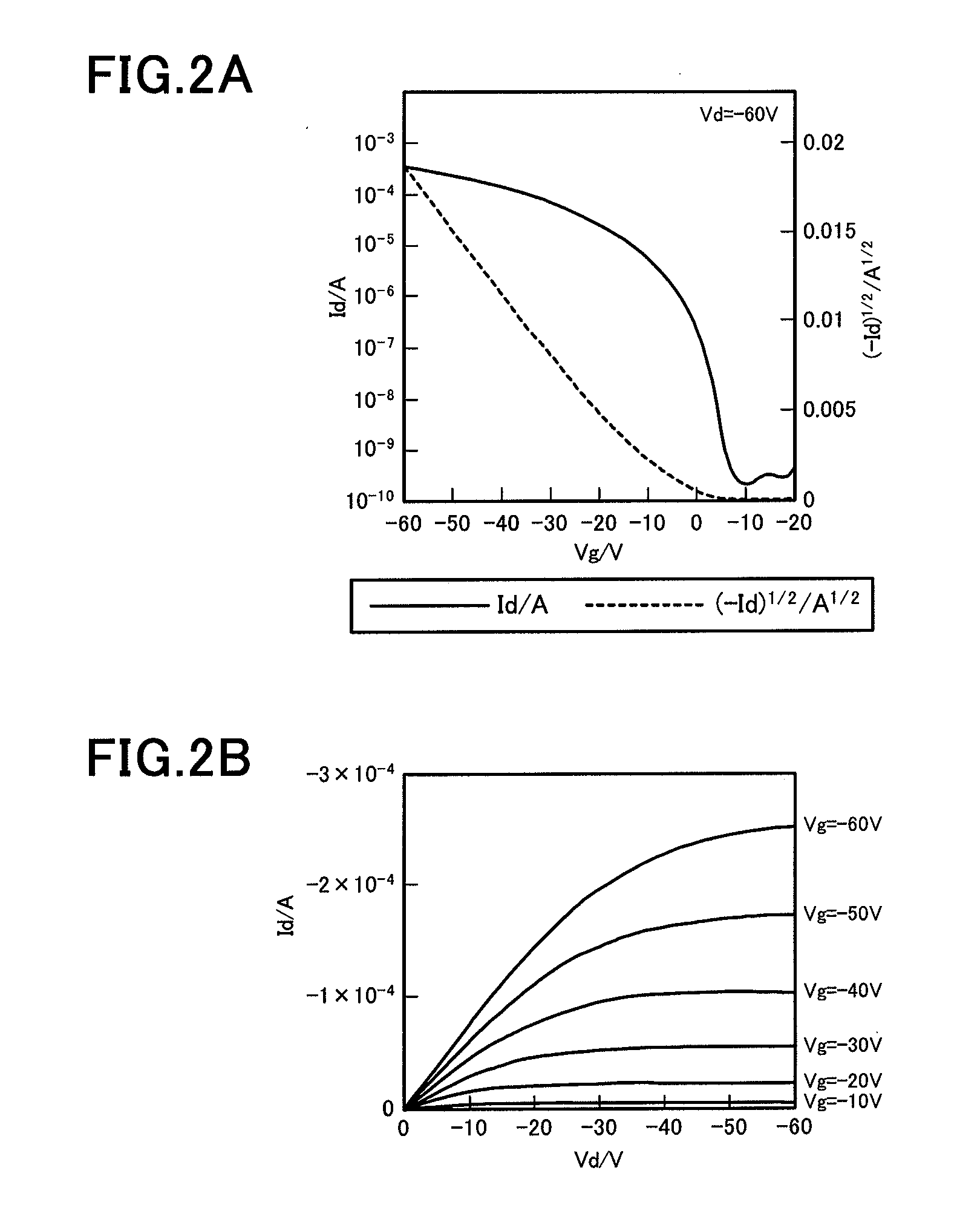
Novel Compound, Method of Producing the Compound, Organic Semiconductor Material and Organic Semiconductor Device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0093]The compound of the present invention and the production method thereof will be explained through specific examples below.
first example
[0094]An explanation will be given of synthesis of the compound represented by the general formula (1) and having the liner structure. Note that the structure of the compound is set through 1H NMR (1H Nuclear Magnetic Resonance spectrum) and EIMS (mass spectrography spectrum). The apparatuses used for measurement of respective spectra are as follows:
[0095]1H-NMR: JEOL Lambda 400 spectrometer[0096]: JEOL EX-270 spectrometer
[0097]EIMS: Shimadzu QP-5050A
[0098]Those apparatuses were also used in other examples to be discussed later.
[0099]An explanation will be given of synthesis of naphtho[2,3-b:6,7-b′]dithiophene step by step.
Synthesis of 1,3,5,7-tetrabromo-2,6-dihydroxynaphthalene
[0100]2,6-dihydroxynaphthalene (2 g, 12.5 mol) was dissolved in acetic acid (60 ml). The acetic acid was used as a solvent. Bromine (2.6 ml. 50.7 mol) was dripped in the solution, and reaction was carried out under a reflux temperature (120° C. to 125° C.).
[0101]As is explained in the embodiment for carrying ...
second example
[0135]Next, an explanation will be given of synthesis of 2,7-diphenylnaphto[2,3-b:6,7-b′]dithiophene step by step.
Synthesis of 2,6-dibromo-3,7-bis(phenylethynyl)naphthalene
[0136]Using 3,7-dibromo-2,6-bis(trifluoromethanesulfonyl)naphthalene synthesized through the above-explained method, 2,6-dibromo-3,7-bis(phenylethynyl)naphthalene was synthesized through the following procedures.
[0137]Under a nitrogen atmosphere, 3,7-dibromo-2,6-bis(trifluoromethanesulfonyl)naphthalene (582 mg, 1.0 mmol) was dissolved in DMF (7 ml) and diisopropylamine (7 ml). The solution was degassed for 30 minutes.
[0138]Pd(PPh3)2Cl2 (70 mg, 0.05 mmol, 10 mol %) and CuI (38 mg, 0.1 mmol, 20 mol %) as catalysts, and phenylacetylene (0.22 ml, 2.0 mmol) as a reagent were added to the solution, and the solution was stirred for 11 hours at a room temperature in order to let it reacted. Thereafter, pure water (1 ml) and 1N hydrochloric acid (1 ml) were added in order to terminate the reaction.
[0139]The reaction soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com