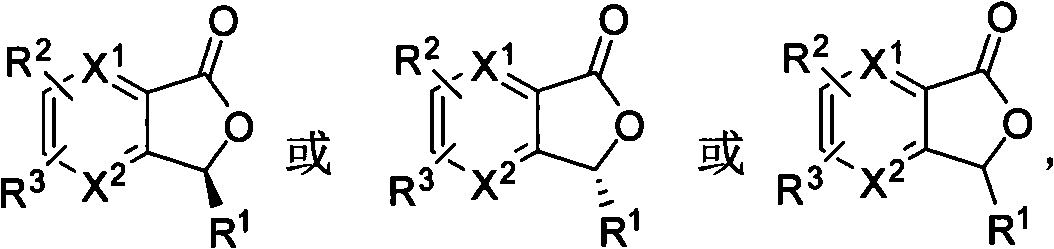
3-substituted benzene phthalein compounds with biological activity
A compound and phthalide technology, applied in the field of 3-substituted phthalide compounds, can solve problems such as no effective treatment yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0021] Preparation of embodiment 13-substituted phthalides:
[0022]The 3-substituted phthalides involved in the present invention can be prepared according to the following two methods, wherein method one is used to prepare chiral novel 3-substituted phthalides, and method two is used to prepare racemic novel 3-substituted phthalides.
[0023] Method 1: From room temperature to 80°C, with a substrate concentration of 0.5-1.25 mol / L, in 2-5 equivalents of reducing agent formate, chiral diamine ligand and (p-methylisopropyl)phenyl dichloride Under the catalysis of the complex catalyst of ruthenium dimer, the number of moles of the substrate and the number of moles of the catalyst are 100-500:1, react for 4-24 hours, and purify by column chromatography to obtain a certain yield and a certain optical purity Product; General reaction formula is as follows:
[0024]
[0025] Among them, the main configuration of the product is determined by the configuration of the chiral liga...
Embodiment 2
[0037] Embodiment 2 biological experiments:
[0038] 1. Drosophila training and learning index measurement operation of olfactory short-term memory deficit test:
[0039] About 100 fruit flies were placed in an automatic training device for training. During the training, two gases 1.5‰ octanol and 1‰ methylcyclohexanol were introduced successively, one with electric shock CS+ and the other without electric shock CS-, 1 Their memory was tested immediately after the period training ended. During the detection, the fruit flies were placed in the center of two opposite smells, and allowed to choose freely for 120 seconds, and the learning index was calculated according to the number of fruit flies that chose each smell.
[0040] 2. Drosophila training and learning index measurement operation of the olfactory long-term memory deficit test:
[0041] About 100 fruit flies were trained in an automatic training device. During the training, two gases 1.5‰ octanol and 1‰ methylcyclohex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com