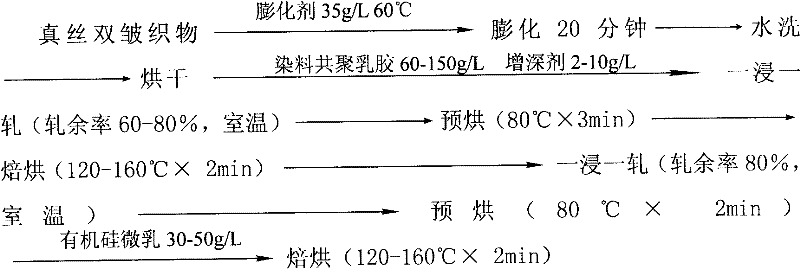
Azo dye acrylate, its copolymer latex paint dyeing water-based adhesive and preparation method thereof
A water-based adhesive and acrylate technology, which is applied in the field of its copolymer latex paint dyeing water-based adhesive and azo dye acrylate, can solve the problems of printing and dyeing wastewater, which are unfavorable for environmental protection, reducing strength, and damaging fibers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
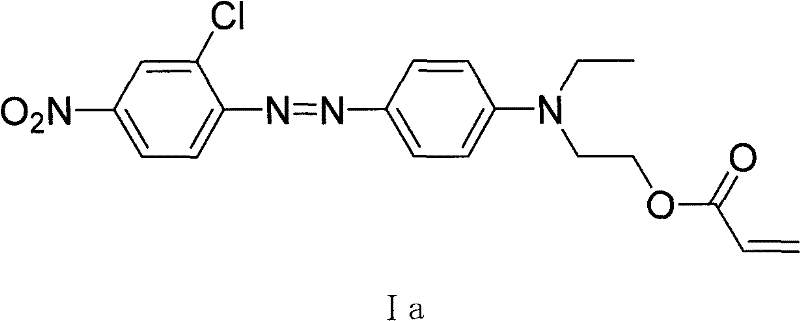
[0054] Example 1: Preparation of 2-chloro-4-nitro-4'-N, N-ethyl hydroxyethylaminoazophenyl acrylate (Ia)
[0055] Add 2-chloro-4-nitro-4'-(N-ethyl-N-β-hydroxyethyl ) 104.5 g (0.30 mol) of aminoazobenzene and 500 ml of dry acetone, stirred for 20 minutes, heated to 30 ° C, and slowly dropped in 40.7 g (0.45 mol) of acryloyl chloride with a constant pressure dropping funnel, and the dropwise addition was completed in about one hour . TLC tracking. After the reaction was completed, the system was cooled to 0° C., and under stirring, 300 ml of water was added to stop the reaction, and a large amount of yellow precipitates appeared. The yellow crude product is isolated by suction filtration, washed with ethanol and dried. Then recrystallize with acetone / ethanol to obtain 103 g of yellow powder, yield 85.3%. 1 H-NMR (ppm) δ: 8.41 (3H, m; 3, 5, 6-ArH); 8.15 (2H, m; 3', 5'-ArH); 7.03 (2H, m; 2', 6'- ArH); 6.43~5.80 (3H, m; CH 2 =CH-); 4.38 (2H, t; -OCH2-); 3.39 (2H, q; -CH2-); 1...
Embodiment 2
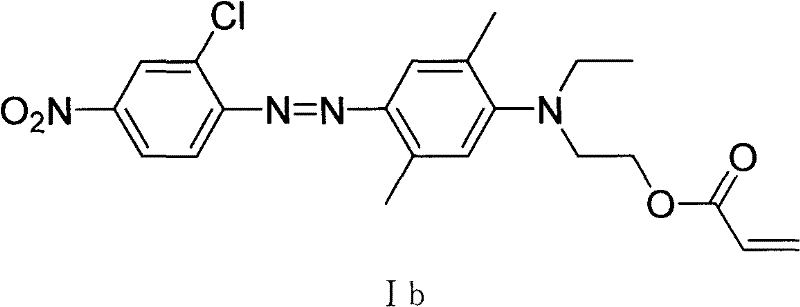
[0057] Example 2: Preparation of 2-chloro-4-nitro-2', 5'-dimethyl-4'-N, N-ethyl hydroxyethylaminoazophenyl acrylate (Ib)
[0058] This example is basically the same as Example 1, except that 2-chloro-4-nitro-2', 5'-dimethyl-4'-(N-ethyl-N-β-hydroxyethyl 113 grams (0.30mol) of aminoazobenzene 113 grams (0.30mol) replace above-mentioned raw material. 108 g of red powder were obtained with a yield of 83.6%. 1 H-NMR (ppm) δ: 8.43 (1H, m; 3-ArH); 8.11 (2H, m; 5, 6-ArH); 7.43 (1H, s; 3'-ArH); 6.47 (1H, s; 6'-ArH); 6.40~5.78(3H, m; CH 2 =CH-); 4.40 (2H, t; -OCH2-); 3.67 (2H, t; NCH2-); 3.40 (2H, q; -CH2-); 2.35 (6H, s; 2′, 5′-ArCH 3 ); 1.15(3H,t; -CH 3 ). Its structural formula is:
[0059]
Embodiment 3
[0060] Example three: Preparation of 2-chloro-4-nitro-6-cyano-2′-methyl-5′-acetylamino-4′-N, N-ethyl hydroxyethylaminoazophenyl acrylate ( Ic)
[0061] This example is basically the same as Example 1, except that 2-chloro-4-nitro-6-cyano-2'-methyl-5'-acetylamino-4'-(N-ethyl- N-hydroxyethyl) aminoazobenzene 133.4 grams (0.30mol) replaces the raw material among the embodiment one. 120.7 g of blue powder was obtained with a yield of 80.7%. 1 H-NMR (ppm) δ: 10.01 (1H, br; 5′-ArNHCO); 8.68 (1H, d; 3-ArH); 8.52 (1H, d; 5-ArH); 7.53 (1H, s; 3′ -ArH); 7.05 (1H, s; 6'-ArH); 6.38~5.72 (3H, s; CH 2 =CH-); 4.31 (2H, t; -OCH2-); 3.61 (2H, t; NCH2-); 3.39 (2H, q; -CH2-); 2.30 (3H, s; 2'-ArCH3); 2.02 (3H, s; CH3CO-); 1.10 (3H, t; -CH3).
[0062]
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com