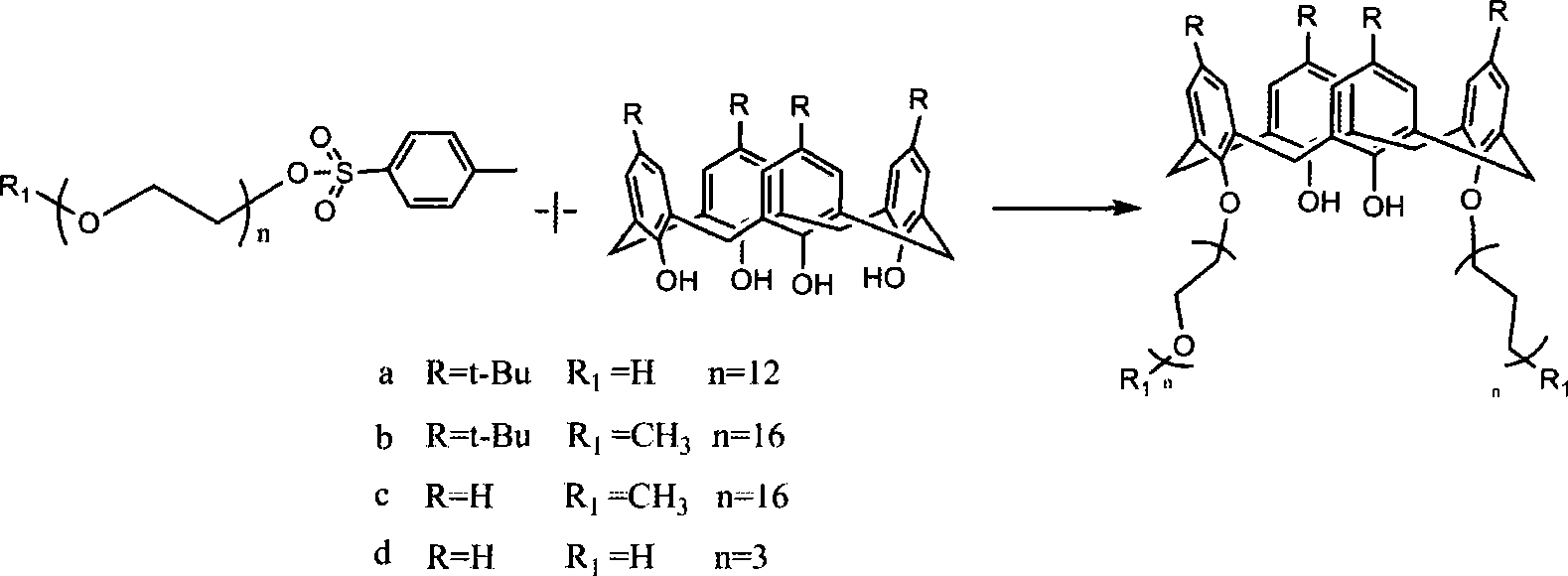Preparation method for polyglycol modified amphipathic calixarene
A technology of polyethylene glycol and calixarene, which is used in the preparation of ether, the preparation of ether by ester reaction, and organic chemistry, etc. problem, to achieve the effect of high yield and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step a: In a 250 ml round bottom flask, add 5.2 g of end-methyl polyethylene glycol 550, 780 mg of sodium hydroxide, add 40 ml of water and stir to dissolve. 2.4 g of p-toluenesulfonyl chloride was dissolved in 40 ml of tetrahydrofuran, and the p-toluenesulfonyl chloride solution was added dropwise into the reaction flask with a constant pressure dropping funnel, and the addition was completed within 2 hours. After reacting in the refrigerator for 4 hours, the crude product was extracted with dichloromethane, and washed three times with deionized water to remove excess alkali, unreacted raw materials and by-products. When the molecular weight of polyethylene glycol is greater than 1000, it can be further purified by recrystallization from anhydrous ether. Final yield 85%.
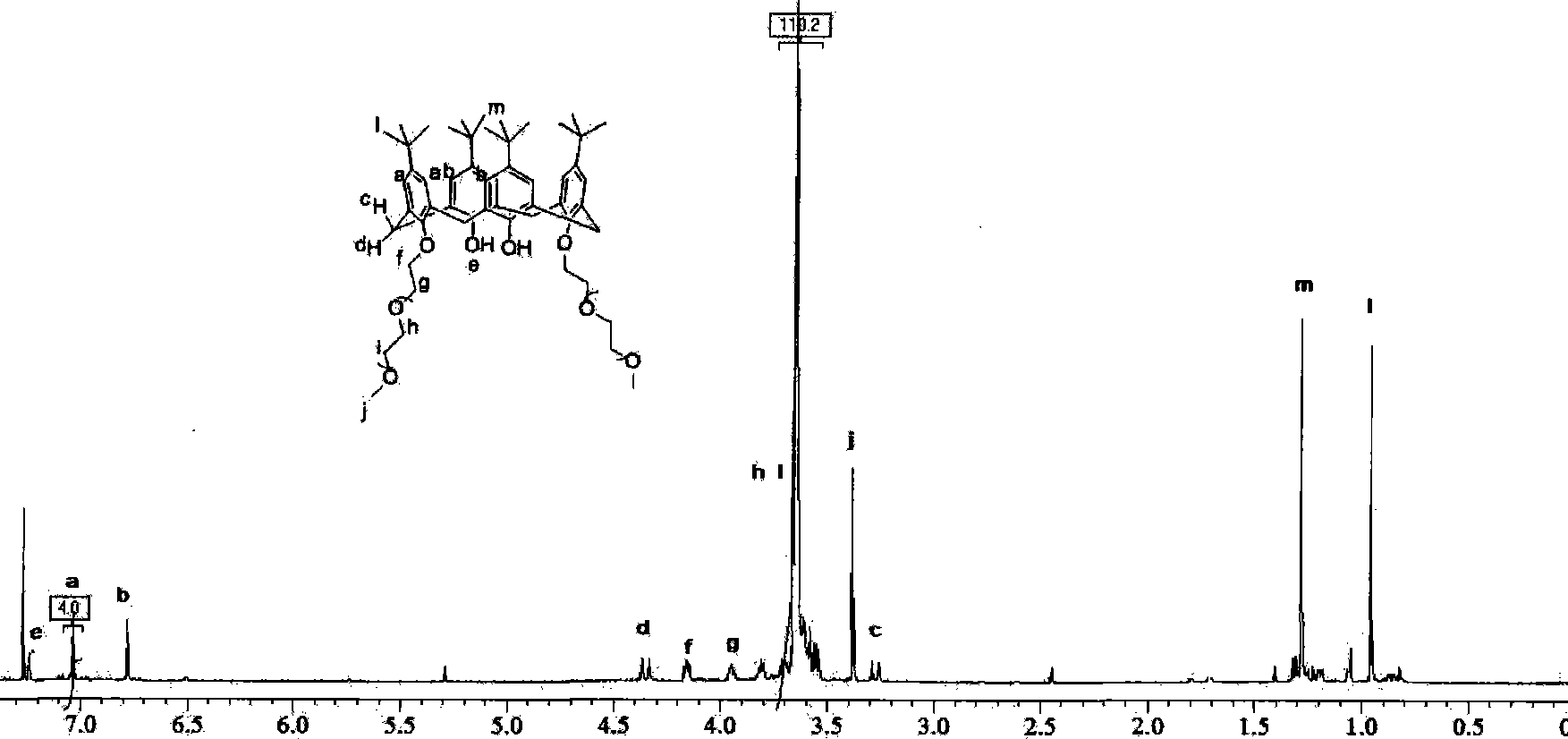
[0025] Step b: In a 250 ml three-neck flask, add 1.29 g of tert-butylcalix[4]arene, 2.65 g of end-methyl polyethylene glycol 550, 559 mg of potassium carbonate, and 70 ml of anhydrous acetonitrile. ...
Embodiment 2
[0027] Step a: Step a: In a 250 ml round bottom flask, add 7.1 g of end-methyl polyethylene glycol 750, 780 mg of sodium hydroxide, add 50 ml of water and stir to dissolve. 2.4 g of p-toluenesulfonyl chloride was dissolved in 40 ml of tetrahydrofuran, and the p-toluenesulfonyl chloride solution was added dropwise into the reaction flask with a constant pressure dropping funnel, and the addition was completed within 2 hours. After reacting in the refrigerator for 4 hours, the crude product was extracted with dichloromethane, and washed three times with deionized water to remove excess alkali, unreacted raw materials and by-products. When the molecular weight of polyethylene glycol is greater than 1000, it can be further purified by recrystallization from anhydrous ether. The final yield was 78%.
[0028]Step b: In a 250 ml three-necked flask, add 1.29 g of tert-butylcalix[4]arene, 3.67 g of terminal methyl polyethylene glycol 750, then add 559 mg of potassium carbonate as a ca...
Embodiment 3
[0030] Step a: same as embodiment 1 step a
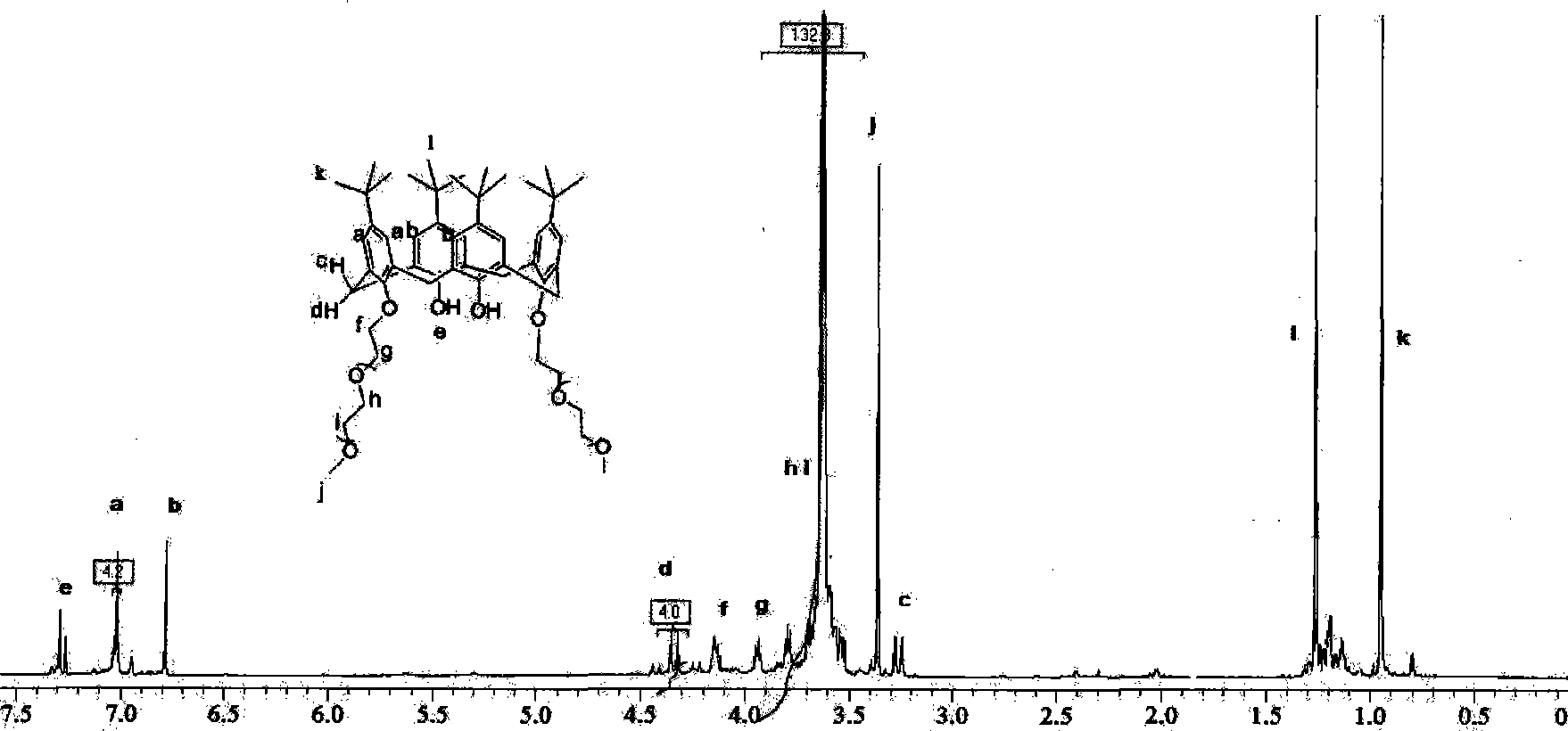
[0031] Step b: In a 250 ml three-necked flask, add 0.85 g of de-tert-butylcalix[4]arene, 2.65 g of end-methyl polyethylene glycol 550, 559 mg of potassium carbonate, and 70 ml of anhydrous acetonitrile. Stir to dissolve completely, and reflux for two days under nitrogen protection until the calixarene raw material disappears through thin-layer chromatography analysis. Add 20 ml of 0.1 mol / L sodium hydroxide solution and stir for two hours. Acetonitrile was distilled off under reduced pressure, and then 100 ml of ethyl acetate was added for extraction. After the ethyl acetate was evaporated to dryness, the product became a pale yellow oil, 2.8 g of the crude product, and the yield was 80%. The column was chromatographed on silica gel, eluting with dichloromethane / methanol=35:1 to 5:1 gradient, and after purification, 2.2 g of the product was obtained with a yield of 61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com