Histidine-containing polypeptide compound modified by c-h alkylation promoted by visible light and preparation method thereof
A compound and visible light technology, applied in the field of histidine-containing polypeptides and their preparation, to achieve good compatibility and avoid the effect of reducing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0081] Screening of reaction solvents:
[0082]
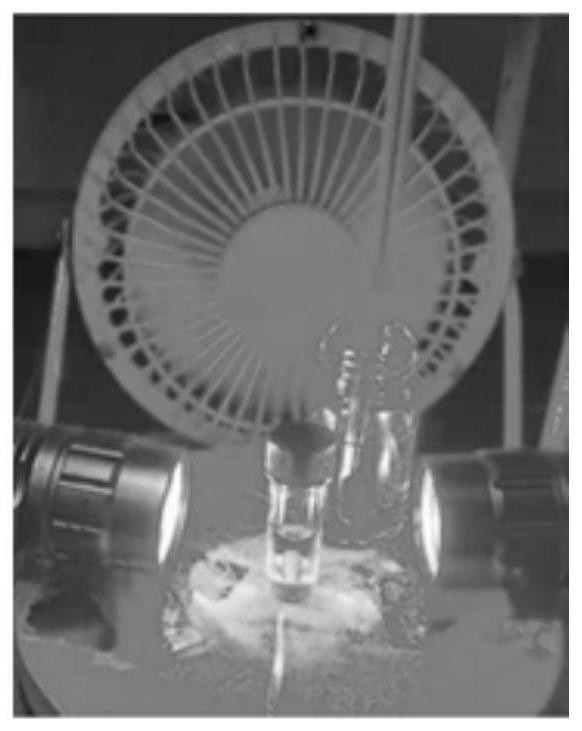
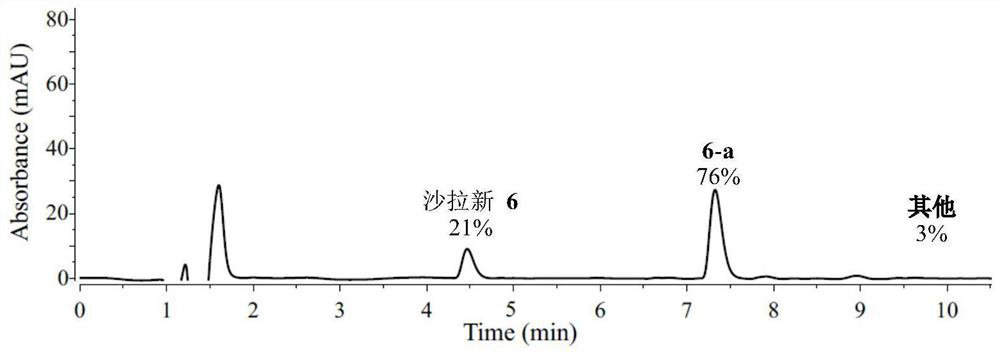
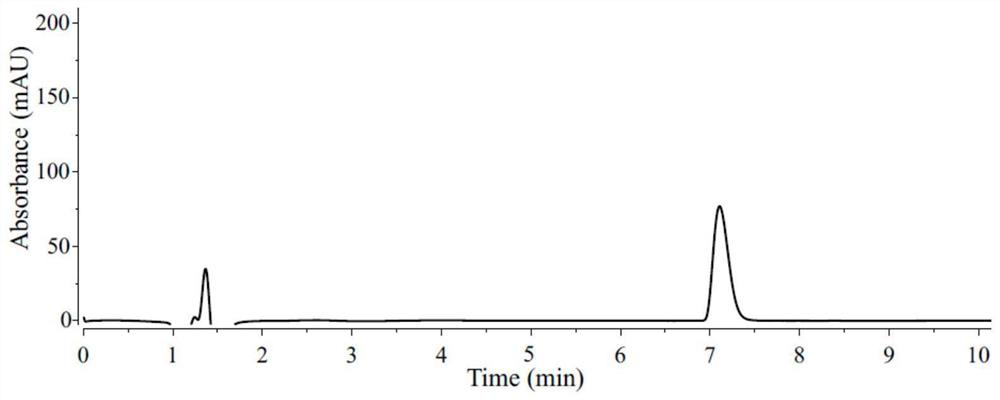
[0083] Peptide 6 (2.0 μmol, 1 equiv.), DHP-a (20.0 μmol, 10 equiv.), deoxygenated TFA (1.49 μL, 20.0 μmol, 10 equiv.) and deoxygenated solvent (0.2 mL , 10 mM) into a 1 mL dry micro-reaction vial equipped with a magnetic stirrer, sealed and taken out from the argon atmosphere glove box. The schematic diagram of the preparation of the reaction is shown in figure 1 As shown, irradiate with two 10W blue LED lamps (the distance between the sample and the lamp is 3cm, and the cooling fan keeps the temperature at about 35°C) for 3 hours, add glacial ether to the reaction solution, and carefully discard the upper liquid after centrifugation. After drying under pressure, LCMS analysis was performed. The second and third cycles were carried out in the same manner as Example 5. The difference of embodiment 1-10 is only the difference of reaction solvent, specifically as shown in table 1, product 6-a structural analysis is as follow...
Embodiment 17-20
[0096] Screening of reagent equivalents in reactions:
[0097]
[0098] Peptide 6 (2.0 μmol, 1 equiv.), DHP-a, deoxygenated TFA and deoxygenated TFE (0.2 mL, 10 mM) were added to a 1 mL dry microreaction vial equipped with a magnetic stir bar in the glove box , sealed and taken out from the argon atmosphere glove box. Irradiate with two 10W blue LED lamps (the sample is 3cm away from the lamp, and the cooling fan keeps the temperature at about 35°C) for 3 hours. Glacial diethyl ether was added to the reaction solution, the upper layer liquid was carefully discarded after centrifugation, and the precipitate was dried under reduced pressure for LCMS analysis. The difference between Examples 17-20 is that the equivalents of DHP-a and TFA used in the reaction are different, as shown in Table 3.
[0099] The screening of DHP-a and TFA equivalent in table 3 reaction
[0100]
[0101] a: LCMs Yleld
[0102] It can be seen from the above results that the use of different equ...
Embodiment 21-22
[0104] Screening of reaction concentration:
[0105]
[0106] In the glove box, peptide 6 (2.0 μmol, 1 equiv.), DHP-a (20.0 μmol, 10 equiv.), deoxygenated TFA (1.49 μL, 20.0 μmol, 10 equiv.) and deoxygenated TFE were added to Put it in a 1mL dry micro reaction vial equipped with a magnetic stirrer, seal it and take it out of the argon atmosphere glove box. Irradiate with two 10W blue LED lamps (the sample is 3cm away from the lamp, and the cooling fan keeps the temperature at about 35°C) for 3 hours. Glacial diethyl ether was added to the reaction solution, the upper layer liquid was carefully discarded after centrifugation, and the precipitate was dried under reduced pressure for LCMS analysis. The difference between Examples 21-22 is only the difference in reaction concentration, as shown in Table 4.
[0107] Screening of table 4 reaction concentration
[0108]
[0109] a: LCMS Yield
[0110] From the above results, it can be seen that the reaction concentration has ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com