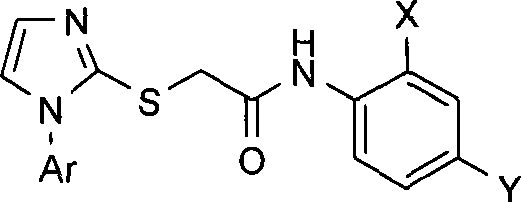
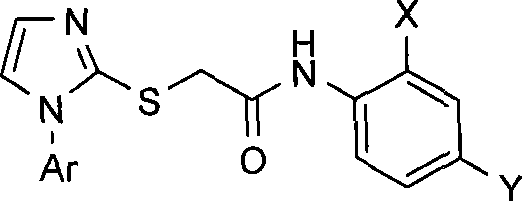
N-substituted phenyl-2-(1-aryl-1H-imidazolyl-2-sulfhydryl) acetamides derivates, preparation method thereof and application
A technology of acetamide and derivatives, applied in the field of anti-HIV drugs, can solve problems such as metabolic instability in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. Preparation of intermediate 1-(4-chlorophenyl)-1H-imidazole-2-mercapto (3a)
[0032] Dissolve 8.5g (0.05mol) of p-chlorophenylisothiocyanate (1a) in 100ml of EtOH, add 5.3g (0.05mol) of aminoacetaldehyde dimethyl acetal dropwise under stirring at room temperature, and stir for 30min after dropping, the reaction is exothermic , a large amount of white solids were precipitated, the reaction liquid was cooled, suction filtered, washed with cold EtOH, and 1-(4-chlorobenzene)-3-(2,2-dimethoxyethyl)thiourea (2a) crude product was obtained, no purification was necessary , proceed directly to the next reaction. The crude product was heated to reflux with 50ml 5N HCl for 3h, cooled, the reaction solution was adjusted to be alkaline with 4N NaOH solution, filtered with suction, the filtrate was acidified with concentrated hydrochloric acid, a large amount of white solid was precipitated, washed with water, and dried to obtain 1-(4-chlorobenzene )-1H-imidazole-2-mer...
Embodiment 2
[0033] Example 2. Preparation of N-(2-chlorophenyl)-2-(1-(4-chlorophenyl)-1H-imidazole-2-mercapto)acetamide (5a1)
[0034] Add 0.08g (0.002mol) NaOH to the suspension of 0.42g (0.002mol) 1-(4-chlorobenzene)-1H-imidazole-2-mercapto (3a) in EtOH (30ml), stir at room temperature, and wait until the suspension After the liquid is clarified, add 0.40 g of equivalent 2-chloroacetyl-N-o-chloroaniline (4.1), continue to stir at room temperature, TLC detects that the reaction is complete, and evaporate the solvent to dryness under reduced pressure to obtain an off-white solid, to which 30 ml of dichloromethane is added , washed with 3×30ml water, the organic layer was dried, concentrated, and recrystallized from EtOH to obtain 0.37g of the target compound (5a1), white needle-like crystals, yield: 48.9%, mp: 233.1-235.0°C.
[0035] Product spectral analysis data:
[0036] 1 H-NMR (CDCl 3 , ppm) δ: 10.54 (s, 1H, NH), 8.33 (d, 1H, J=7.8Hz), 7.46 (d, 2H, J=8.4Hz, PhH), 7.35 (dd, 1H, J ...
Embodiment 3
[0040] Example 3. Preparation of N-(2-nitrophenyl)-2-(1-(4-chlorophenyl)-1H-imidazole-2-mercapto)acetamide (5a2)
[0041] Method as described in Example 2, the difference is to add 0.10g (0.001mol) Na to the EtOH (30ml) suspension of intermediate reagent 3a0.42g (0.002mol) prepared in Example 1 2 CO 3 , stirred at room temperature, and after the suspension was clarified, 0.43 g of an equivalent amount of 2-chloroacetyl-N-o-nitroaniline (4.2) was added, and continued to stir at room temperature. TLC detected that the reaction was complete, and the solvent was evaporated to dryness under reduced pressure to obtain a yellow solid , adding 30ml of dichloromethane to it, washing with 3×30ml of water, drying the organic layer, concentrating, and recrystallizing with EtOH to obtain 0.30g of the target compound (5a2), light yellow needle-like crystals, yield: 38.7%, mp: 240.1-241.8°C .
[0042] Product spectral analysis data:
[0043] IR (KBr, cm -1 )3156(υ NH ), 1708 (υ -CONH- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com