Method for preparing dissolvable high activity recombinant human epidermal growth factor
A high-activity technology of epidermal growth factor, applied in the field of preparation of soluble high-activity recombinant human epidermal growth factor, can solve the problems of low yield and activity, low purity of human epidermal growth factor, etc., and achieve simple production process and simplified purification steps , The effect of stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
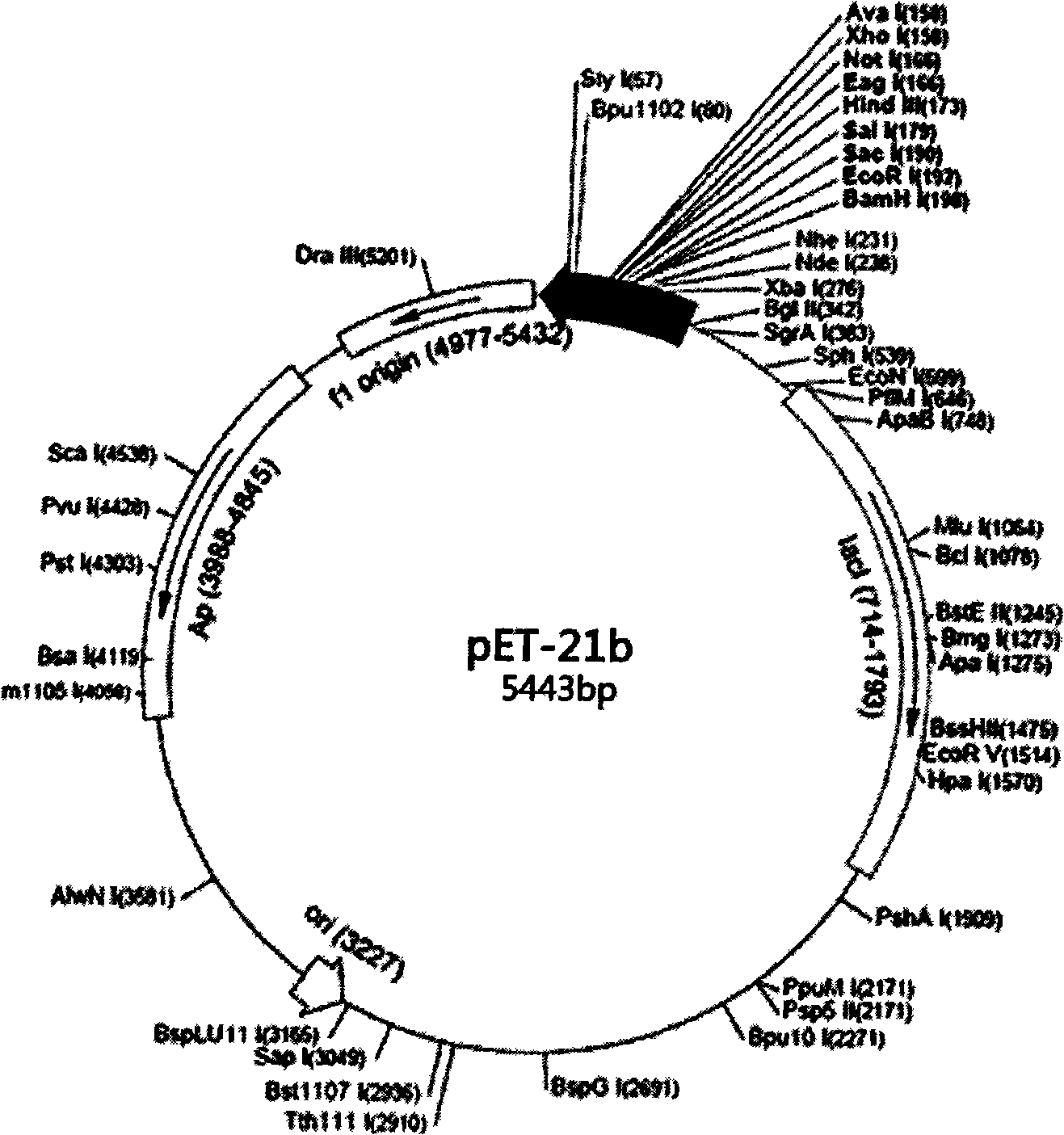
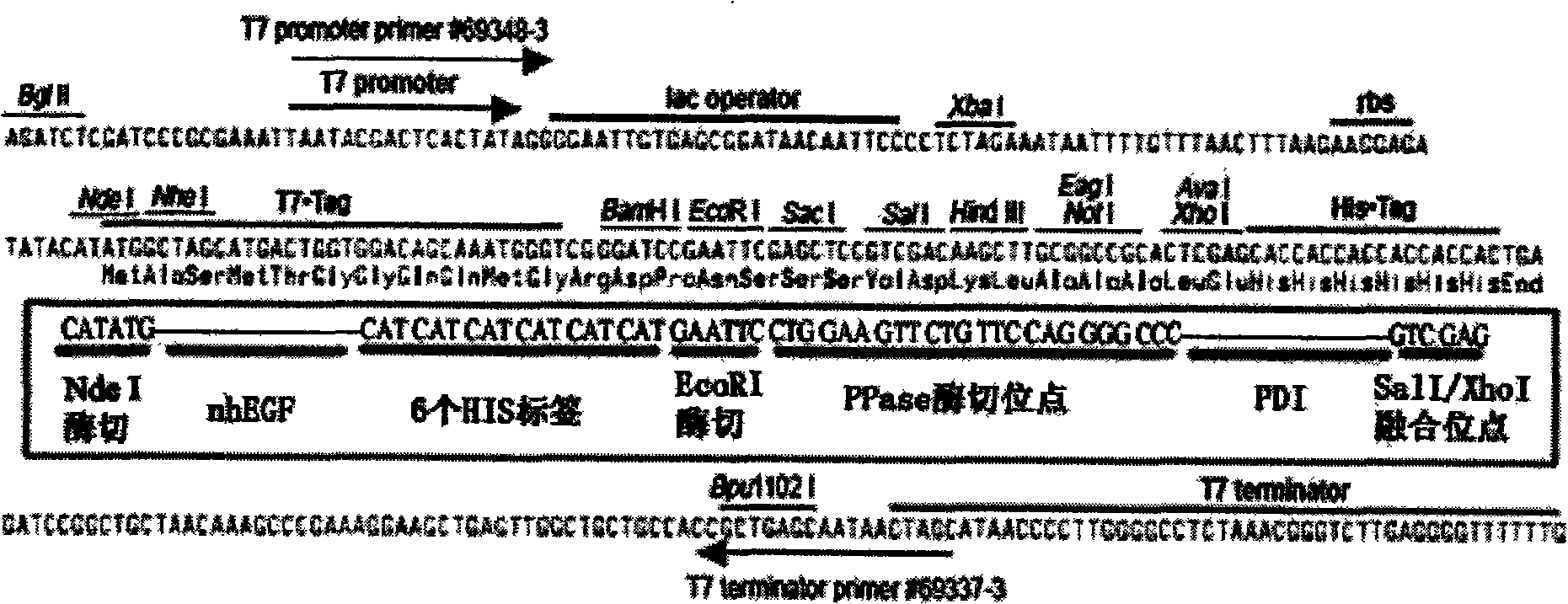
[0017] Embodiment one: Construction and expression of hEGF gene expression vector:
[0018] The full-length sequence of human epidermal growth factor (hEGF) is as follows:
[0019] AATAGTGACTCTGAATGTCCCCTGTCCCACGATGGGTACTGCCTCCATGATGGTGTGTGCATGTATATTGAAGCATTGGACAAGTATGCATGCAACTGTGTTGTTGGCTACATCGGGGAGCGATGTCAGTACCGAGACCTGAAGTGGTGGGAACTGCGC
[0020] And replace the codons unfavorably expressed in E. coli to obtain the optimized gene sequence nhEGF:
[0021] AACTCAGATTCCGAATGTCCGCTGTCGCATGATGGGTACTGCCTGCATGATGGTGTCTGCATGTACATCGAAGCGCTGGATAAATATGCCTGCAATTGTGTCGTGGGTTACATCGGGGAACGCTGCCAATATCGTGATCTGAAATGGTGGGAATTGCGC
[0022] Design a complete set of primers for the nhEGF sequence, all arranged from 5`-3` as follows:
[0023] hEGF-P1: GCATGATGGGTACTGCCTGCATGATGGTGTCTGCATGTACATCGAAGCGCTGGAT
[0024] hEGF-P2: TTCCCCGATGTAACCCACGACACAATTGCAGGCATATTTATCCAGCGCTTCGATG
[0025] hEGF-P3: CAT GGATCC AACTCAGATTCCGAATGTCCGCTGTCGCATGATGGGTACTGCCTGC
[0026]hEGF-P4: GCGCAATTCCCACCCATTTCAGA...
Embodiment 2
[0067] Example 2: Using the hEGF stock solution obtained above to prepare EGF fine products after purification
[0068] In the high concentration solution containing hEGF, adjust its pH value to 7.0-8.0. Prepare loading buffer:
[0069] MCAC-15MCAC-1000
[0070] 20mM Tris hydrochloride pH10.0 20mM Tris hydrochloride pH10.0
[0071] 0.5M NaCl 0.5M NaCl
[0072] 10% (v / v) glycerin (adjustable to 20%) 10% (v / v) glycerin
[0073] 15mMol / L imidazole 1M imidazole
[0074] Wash the Ni-NTA His-Bind Resin column material with MCAC-15 solution, then co-incubate the column material with hEGF solution, and shake gently at room temperature (preferably on ice) for 30 min. Then put it on the column, use MCAC-50 to elute the impurity protein, and finally use MCAC-300 to elute the target protein. The resulting product was dialyzed overnight to obtain high-purity hEGF fine product. The product can be used for further processing into finished products.
Embodiment 3
[0075] Embodiment three: the identification of the refined EGF after purification
[0076] First use SDS-PAGE to identify the concentration of the expression product:
[0077] Sample treatment solution: SDS 1g, Glycerol 5mL, bromophenol blue (BPB) solid trace, dithiothreitol (DDT) 2.5mL, solution B 25mL, add deionized water to 50mL;
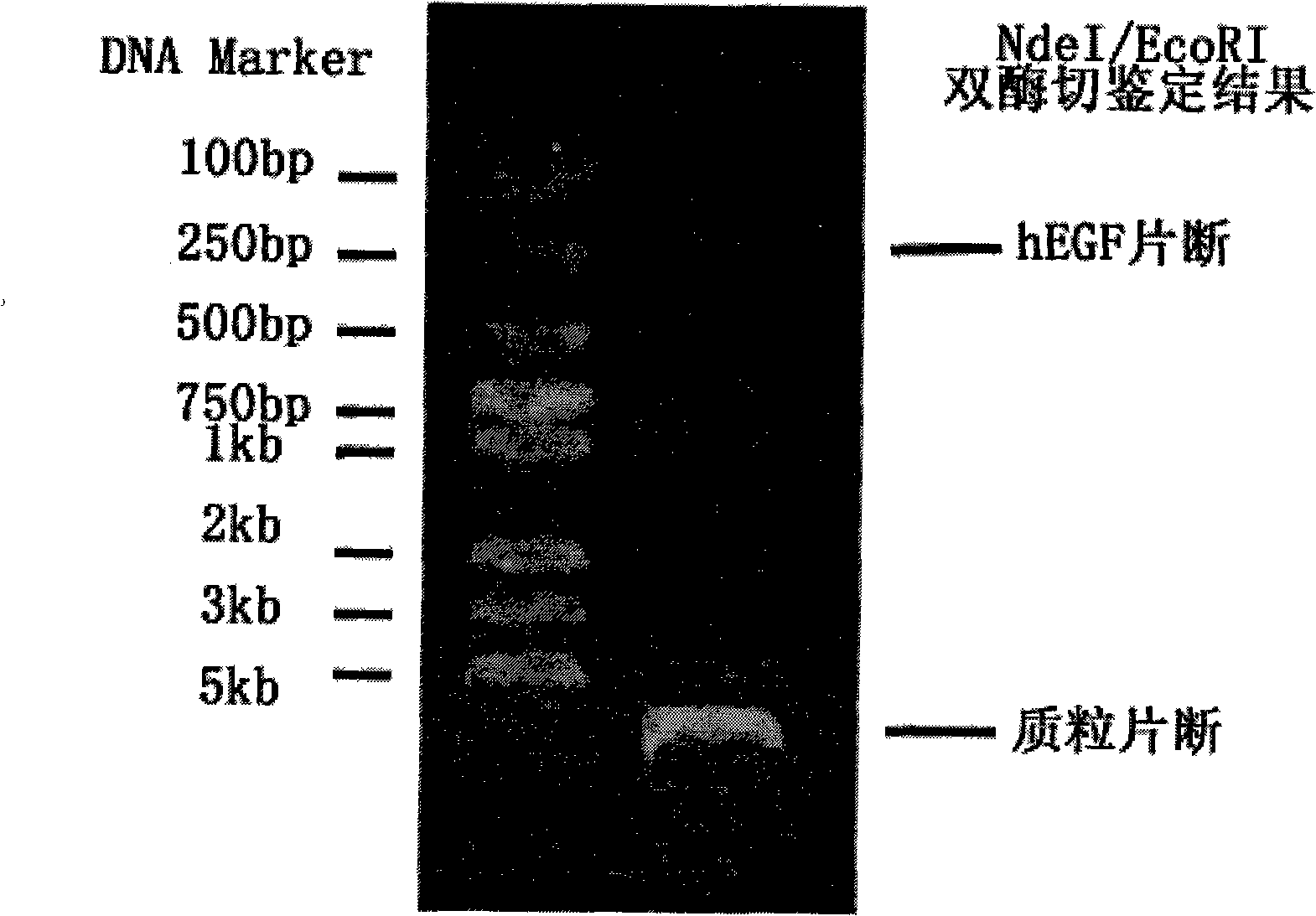
[0078] Take 10ul of the digested hEGF solution, add 2ul of sample treatment solution, place in a boiling water bath for 5 minutes, and apply a sample volume of 5ul / well. The standard protein molecular weight was treated in the same way, stained with Coomassie brilliant blue R-250, and then the concentration was identified by SDS-PAGE. The result is as Figure 4 As shown, the leftmost lane is the standard protein molecular weight control, and the right two lanes are hEGF products refined and purified at different times, showing the purity and concentration of the samples.
[0079] Activity identification of expression products:
[0080]The in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com