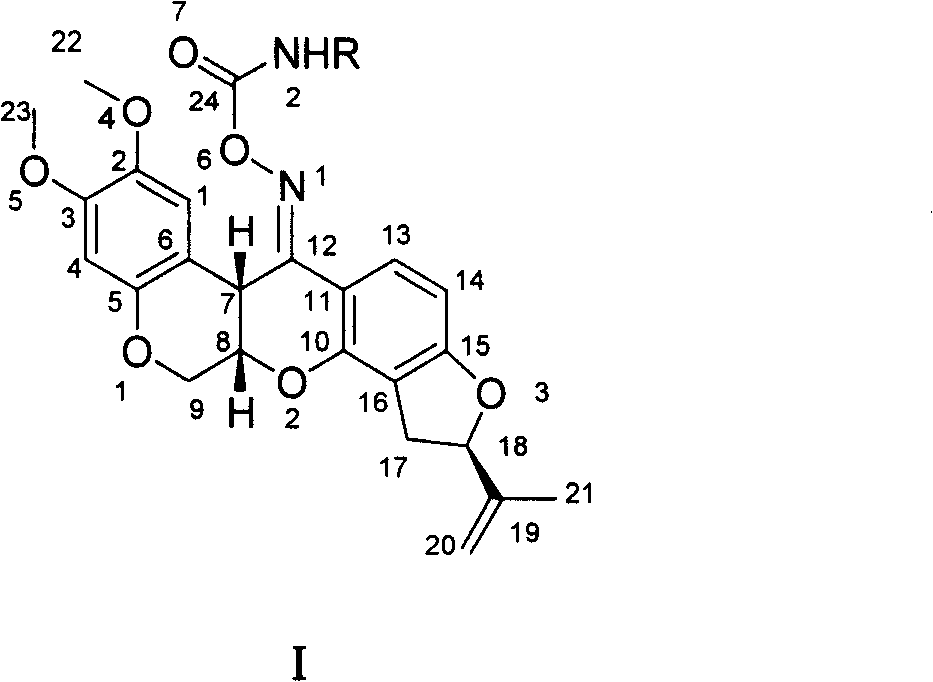
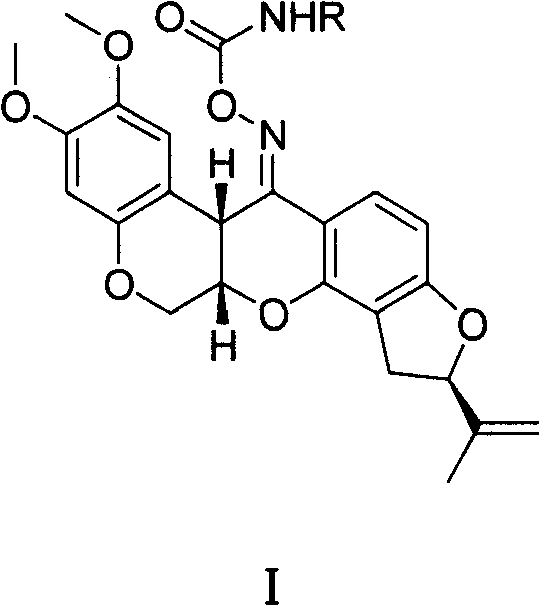
Carbamic tubatoxin oxime ester, preparation and application thereof
A technology for rotenone oxime carbamate and aryl ester, which is applied to the field of new compounds and their preparation, can solve problems such as difficulty in standardization, oxidative decomposition failure, unstable preparation concentration, etc., and achieves short reaction time, improved biological activity, and improved synthesis Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis of embodiment 1 rotenone oxime carbamate (1)
[0019]
[0020] Stir 1.0 g of rotenone oxime with 10.0 ml of acetone or benzene, dissolve, add dropwise 10 ml of acetone solution containing methyl isocyanate, and reflux for 1 hour. TLC monitors the reaction process. The reaction solution was filtered, the filtrate was rotary evaporated to remove the solvent, and the obtained white solid was washed with water. 1 H-NMR (CDCl 3 , 400MHz) δ: 2.75 (s, 3H, CH 3 ), 2.93(dd, J=8.0Hz, J=15.6Hz, 1H, 4'-H), 3.28(dd, J=10.0Hz, J=15.6Hz, 1H, 4'-H), 3.71(s, 3H, OCH 3 ), 3.80 (s, 3H, OCH 3 ), 4.22 (d, J=12Hz, 1H, 6-H), 4.56 (d, J=2.8Hz, 1H, 12a-H), 4.60 (dd, J=12Hz, J=2.8Hz, 1H, 6- H), 4.92(s, 2H, 6a-H, 7'-H), 5.06(s, 1H, 7'-H), 5.17(t, J=8.8Hz, 1H, 5'-H), 6.35( d, J=8.0Hz, 1H, NH), 6.41(s, 1H, 4-H), 6.41(s, 1H, 1-H), 6.48(d, J=8.8Hz, 1H, 10-H), 7.76 (d, J = 8.4 Hz, 1H, 11-H).
Embodiment 2
[0021] The synthesis of embodiment 2 cyclohexylcarbamate rotenone oxime ester (2)
[0022]
[0023] Rotenone oxime 1.0g, acetone or tetrahydrofuran 10.0ml, stir, dissolve, add 10ml acetone solution containing 0.3g cyclohexyl isocyanate, reflux reaction for 7h, add 0.3g cyclohexyl isocyanate, continue reaction for 2h, TLC monitoring reaction process. The reaction liquid was filtered, the filtrate was rotary evaporated to remove the solvent, the white solid obtained was washed with water, and recrystallized from ethanol to obtain 1.0 g of white powder with a melting point of 185-188°C and a yield of 77.0%. 1 H-NMR (CDCl 3 , 400MHz) δ: 1.24~1.44 (m, 4H, c-C 6 h 11 ), 1.64 (m, 4H, c-C 6 h 11 ), 1.76 (s, 3H, 8'-CH 3 ), 1.78 (m, 1H, c-C 6 h 11 ), 2.06(m, 2H, c-C 6 h 11 ), 2.93(dd, J=8.0Hz, J=15.6Hz, 1H, 4'-H), 3.28(dd, J=10.0Hz, J=15.6Hz, 1H, 4'-H), 3.71(s, 3H, OCH 3 ), 3.80 (s, 3H, OCH 3 ), 4.22 (d, J=12Hz, 1H, 6-H), 4.56 (d, J=2.8Hz, 1H, 12a-H), 4.60 (dd, J=12Hz, J...
Embodiment 3
[0024] The synthesis of embodiment 3 octylcarbamic acid rotenone oxime ester (3)
[0025]
[0026] Stir 0.8g of rotenone oxime, 30.0ml of acetone or N-methylpyrrolidone, dissolve, add 0.3g of octyl isocyanate, reflux for 5 hours, add 0.15g of octyl isocyanate, continue the reaction for 10 hours, and monitor the reaction process by TLC. The reaction solution was filtered, the filtrate was rotary evaporated to remove the solvent, the white solid obtained was washed with water, and recrystallized from ethanol to obtain 0.96 g of white powder with a melting point of 140-143° C. and a yield of 86.8%. 1 H-NMR (CDCl 3 , 400MHz) δ: 0.89 (t, J=6.8Hz, 3H, CH 3 ), 1.30(m, 10H, 5×CH 2 ), 1.65 (m, 2H, CH 2 ), 1.76 (s, 3H, 8'-CH 3 ), 2.93(dd, J=8.0Hz, J=16Hz, 1H, 4'-H), 3.29(dd, J=9.6.Hz, J=16Hz, 1H, 4'-H), 3.37(q, J =6.8Hz, 2H, NCH 2 ), 3.71 (s, 3H, OCH 3 ), 3.80 (s, 3H, OCH 3 ), 4.23 (d, J=12Hz, 1H, 6-H), 4.57 (d, J=2.4Hz, 1H, 12a-H), 4.60 (dd, J=12Hz, J=2.4Hz, 1H, 6- H), 4.92...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com