Synthesis of L-benzyl- glutamic ester polymer
A technology of glutamic acid ester and synthesis method, applied in the chemical industry, can solve the problems of difficult bioactive molecule functionalization of polypeptide, inability to prepare polypeptide-based biological materials, and no preparation method is proposed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
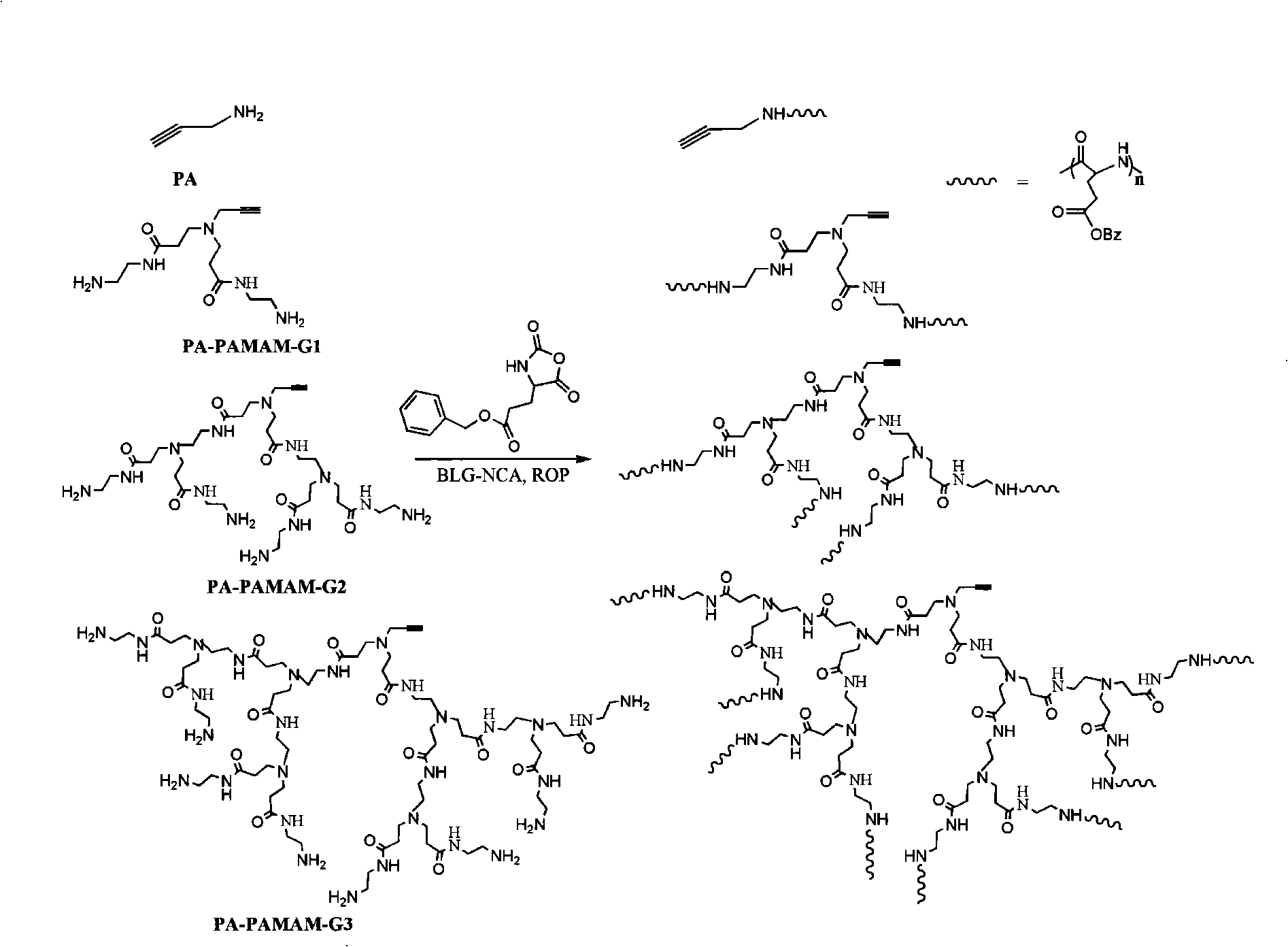
[0013] Example 1: Synthesis of Alkynylated L-Benzyl-Glutamate Polymers from Zero-Generation Alkynylated Amide-Amine Polymers
[0014] Pipette propargylamine (PA, 4.1uL, 0.061mmol) into a 25mL well-dried round-bottomed flask Gamma-benzyl-L-glutamic acid-N-carboxy anhydride (BLG-NCA) monomer (800.5 mg, 3.04 mmol) was added into the round bottom flask and dissolved with 8 mL of anhydrous DMF. After the two flasks were evacuated and blown with nitrogen for 3 times, the BLG-NCA was transferred to the flask containing PA with a vacuum line under a nitrogen atmosphere, and then transferred to an oil bath at 25°C for 48 hours. After the reaction was completed, most of the solvent was removed by rotary evaporation under reduced pressure, and the resulting product was dissolved in THF (4 mL), precipitated in anhydrous ether (50 mL), discarded the supernatant, and dried in vacuo to obtain a linear structure with one arm The alkynylated L-benzyl-glutamate polymer (526.6 mg, yield: 79.0%)...
Embodiment 2
[0015] Example 2: Synthesis of Alkynylated L-Benzyl-Glutamate Polymers from a Generation of Alkynylated Amide-Amine Polymers
[0016] Weigh a generation of alkynylated amide-amine polymer (PA-PAMAM-G1, 3.9mg, 0.014mmol) into a 25mL fully dry round-bottomed flask containing magnetons, dissolve with 1mL of anhydrous DMF; in another 25mL Add γ-benzyl-L-glutamic acid-N-carboxy anhydride (BLG-NCA) monomer (734.0mg, 2.79mmol) into a well-dried round bottom flask, and dissolve it with 7mL of anhydrous DMF. Both flasks are evacuated After blowing nitrogen three times, the BLG-NCA was transferred to a flask containing PA-PAMAM-G1 with a vacuum line under a nitrogen atmosphere, and then transferred to a 25oC oil bath to react for 48 hours. After the reaction was complete, most of the solvent was removed by rotary evaporation, and the resulting product was dissolved in THF (4 mL), precipitated in anhydrous ether (50 mL), discarded the supernatant, and dried in vacuo to obtain a fan-shape...
Embodiment 3
[0017] Example 3: Synthesis of Alkynylated L-Benzyl-Glutamate Polymers from Dialkynylated Amide-Amine Polymers
[0018] Weigh the di-alkynylated amide-amine polymer (PA-PAMAM-G2, 102.0mg, 0.14mmol) into a 25mL fully dry round-bottomed flask containing magnetons, dissolve with 1mL of anhydrous DMF; in another Add γ-benzyl-L-glutamic acid-N-carboxy anhydride (BLG-NCA) monomer (727.2mg, 2.76mmol) into a 25mL fully dry round bottom flask, and dissolve it with 7mL of anhydrous DMF. After vacuuming nitrogen for 3 times, the BLG-NCA was transferred to a flask containing PA-PAMAM-G2 with a vacuum line under a nitrogen atmosphere, and then transferred to a 25oC oil bath for 48 hours. After the reaction was completed, most of the solvent was removed by rotary evaporation under reduced pressure, and the resulting product was dissolved in THF (4 mL), precipitated in anhydrous ether (50 mL), discarded the supernatant, and dried in vacuo to obtain a fan-shaped structure with 4 arms. Alkyny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com