Nifedipine controlled release preparation and preparation method thereof
A technology of controlled-release preparations and nifedipine, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, and medical preparations containing active ingredients. Avoid problems such as the early morning danger hour
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of nifedipine controlled-release preparation
[0046] Prescription (per 1000 tablets):
[0047] Tablet core: Nifedipine 20g, HPMC K100LV15g, HPMC K100M5g, lactose 5g, sodium chloride 20g, crospovidone 5g, silicon dioxide 0.7g, medicinal ethanol 23g;
[0048] Coating layer: HPMC K100LV69g, lactose 161g, magnesium stearate 1.15g, silicon dioxide 2.3g, medicinal ethanol 130.64g.
[0049] Preparation:
[0050] (1) Tablet cores are granulated separately and pressed into tablets (φ5.5mm shallow concave punch);
[0051] (2) The coating layer is granulated separately;
[0052] (3) Press-coated chips (φ9mm shallow concave punch): first fill part of the coating layer particles in the die hole, then place the tablet core in the center of the die hole, then add the remaining coating layer particles, and then press to form a chip-coated chip.
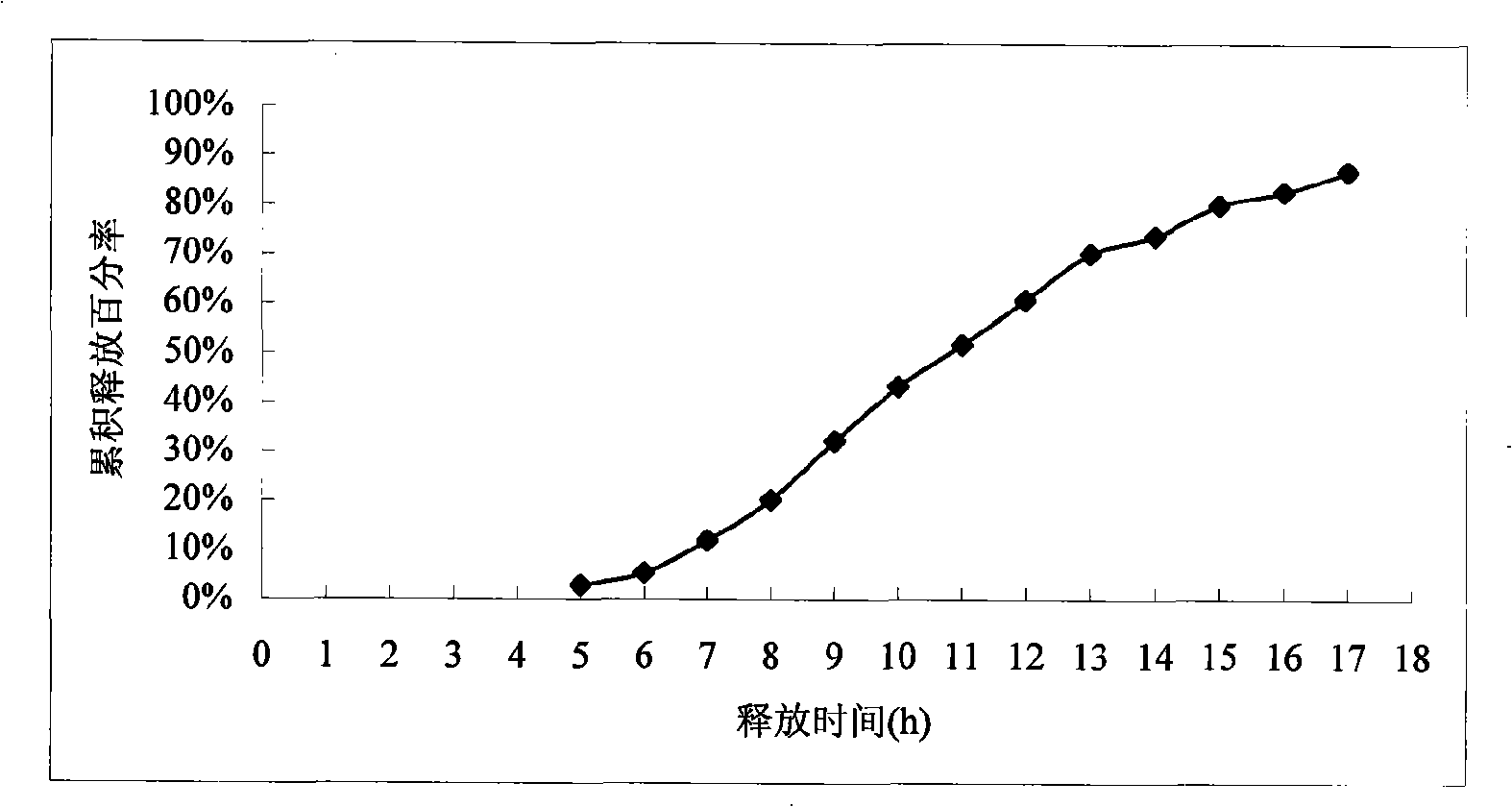
[0053] Determination of in vitro release: according to the United States Pharmacopoeia USP29 nifedipine su...
Embodiment 2
[0055] Embodiment 2: the preparation of nifedipine controlled-release preparation
[0056] Prescription (per 1000 tablets):
[0057] Tablet core: Nifedipine 20g, HPMC K100LV 17.5g, lactose 10g, sodium chloride 17.5g, crospovidone 5g, silicon dioxide 0.7g, medicinal ethanol 18.3g;
[0058] Coating layer: HPMC K100LV69g, lactose 161g, magnesium stearate 1.15g, silicon dioxide 2.3g, medicinal ethanol 130.64g.
[0059] Preparation method: as described under Example 1.
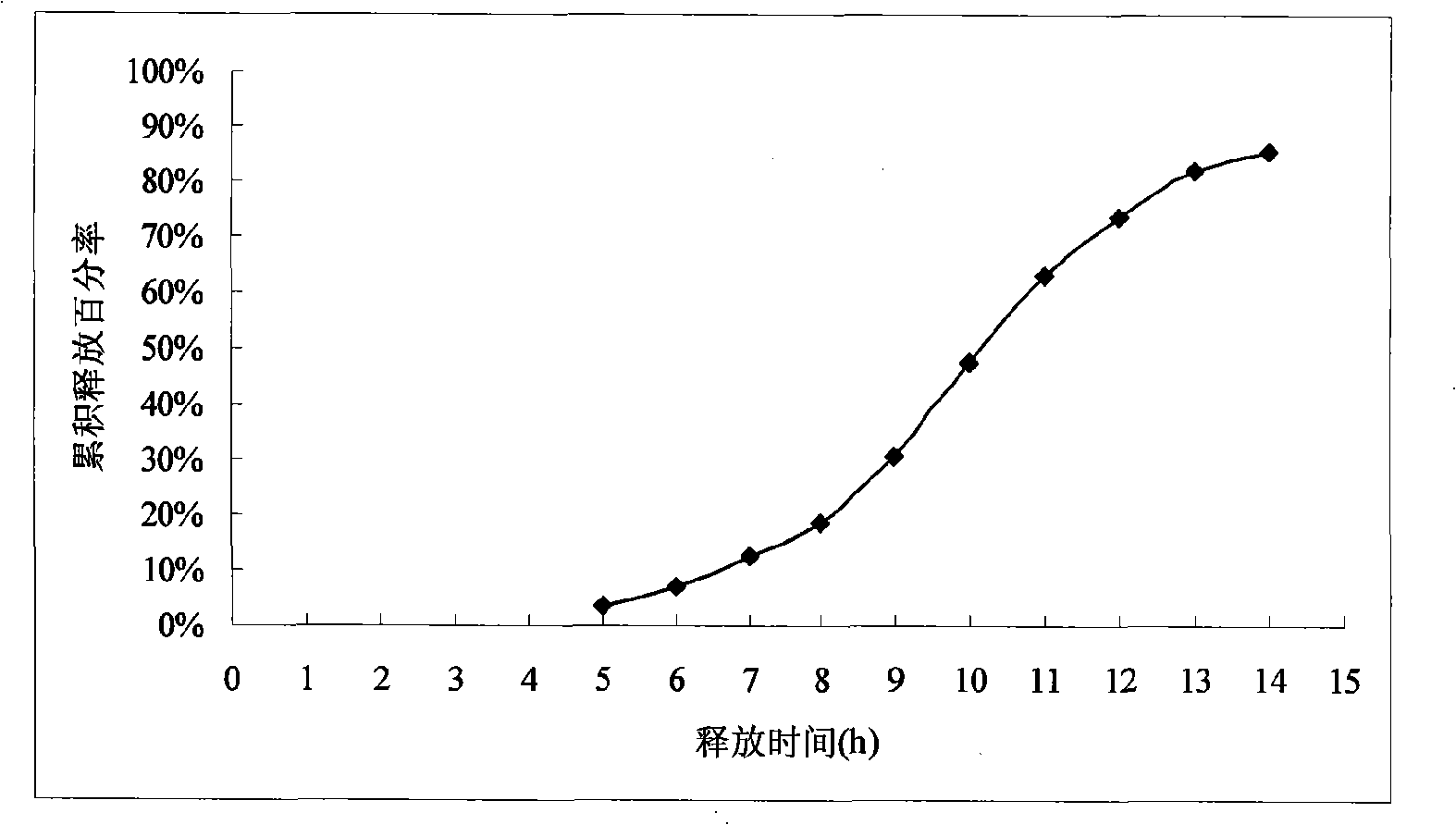
[0060] In vitro release assay: as described under Example 1. (experimental data see table 2 and appendix figure 2 )
[0061] The results showed that the release of the drug started at intervals of 5 to 6 hours, and the sustained release time reached 14 hours.
Embodiment 3
[0062] Embodiment 3: the preparation of nifedipine controlled-release preparation
[0063] Prescription (per 1000 tablets):
[0064] Tablet core: 20g of nifedipine, 35g of HPMC K100LV, 2.5g of lactose, 10g of sodium chloride, 2.5g of crospovidone, 0.7g of silicon dioxide, 22.6g of medicinal ethanol;
[0065] Coating layer: HPMC K100LV69g, lactose 161g, magnesium stearate 1.15g, silicon dioxide 2.3g, medicinal ethanol 130.64g.
[0066] Preparation method: as described under Example 1.
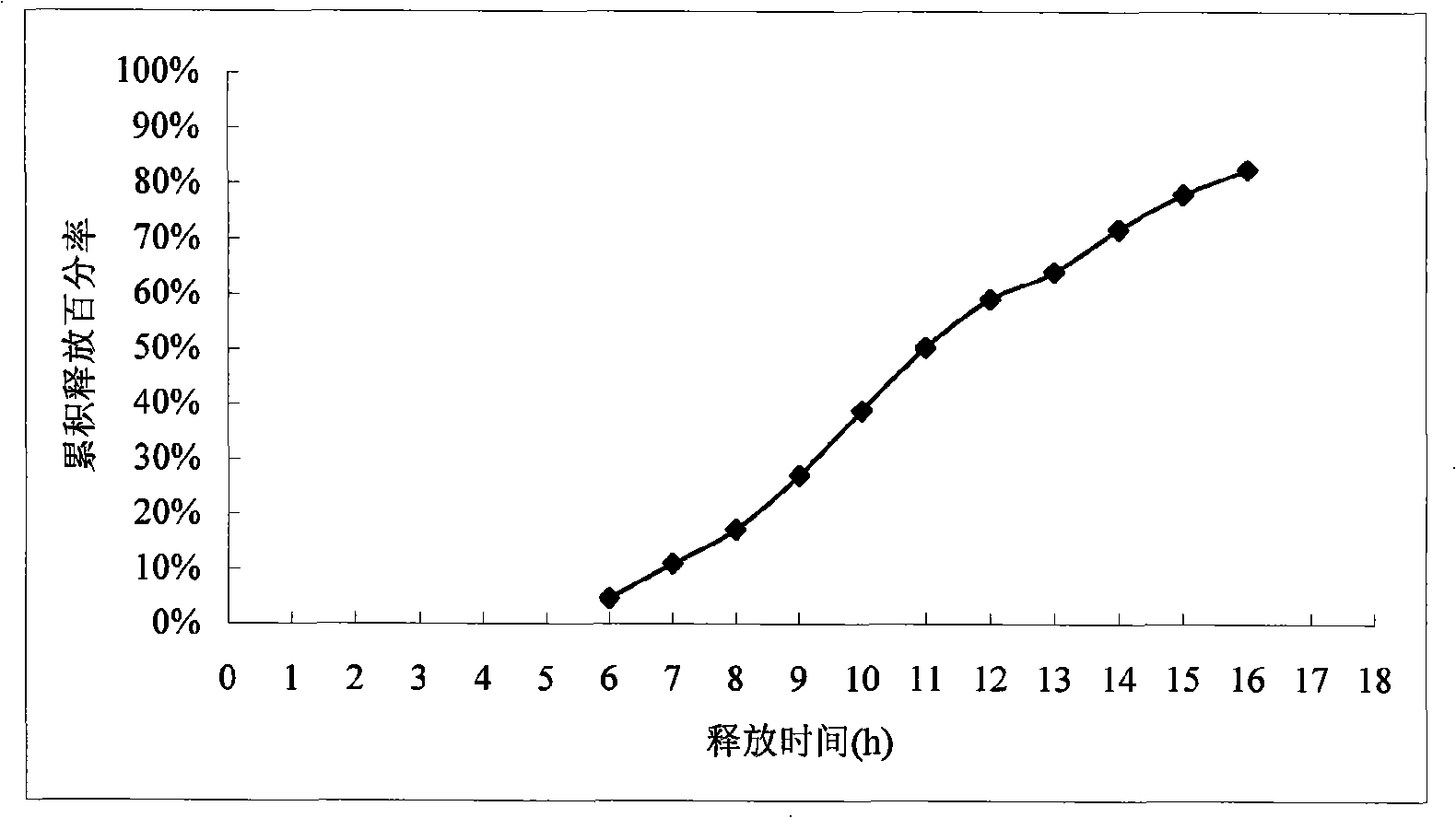
[0067] In vitro release assay: as described under Example 1. (experimental data see table 3 and appendix image 3 )
[0068] The results showed that the release of the drug started at intervals of 6 to 7 hours, and the sustained release time reached 16 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com