Composition containing alkylene oxide derivative of pectin
A kind of rubber alkylene oxide derivatives, technology of alkylene oxide derivatives, applied in the field of pectin alkylene oxide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Embodiment 1: the influence of degree of esterification
[0145] The effect of the degree of esterification was evaluated by detecting the titration curves of the above-mentioned samples. Check the titration curve by the following experimental procedure:
[0146] Titration curve process:
[0147] 1. Dissolve 2 g of pectin in 200 g of deionized water at 70°C and 20°C.
[0148] 2. Place the solution in a water bath with constant temperature control at 25°C and continue stirring.
[0149] 3. 0.1M NaOH was added to the solution and the pH was recorded as a function of 0.1M NaOH added.
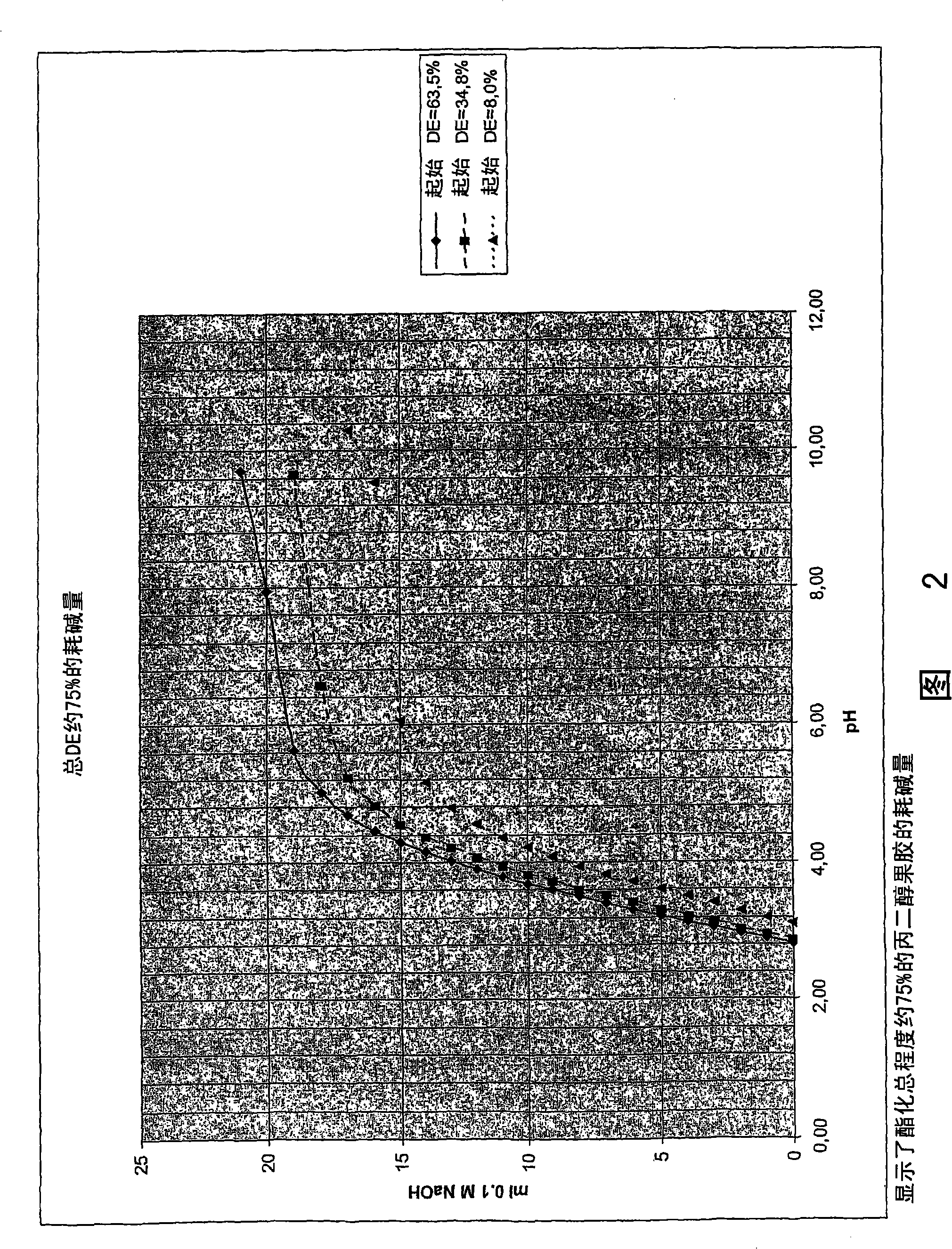
[0150] The results are shown in Table 2 below.
[0151] Table 2
[0152]
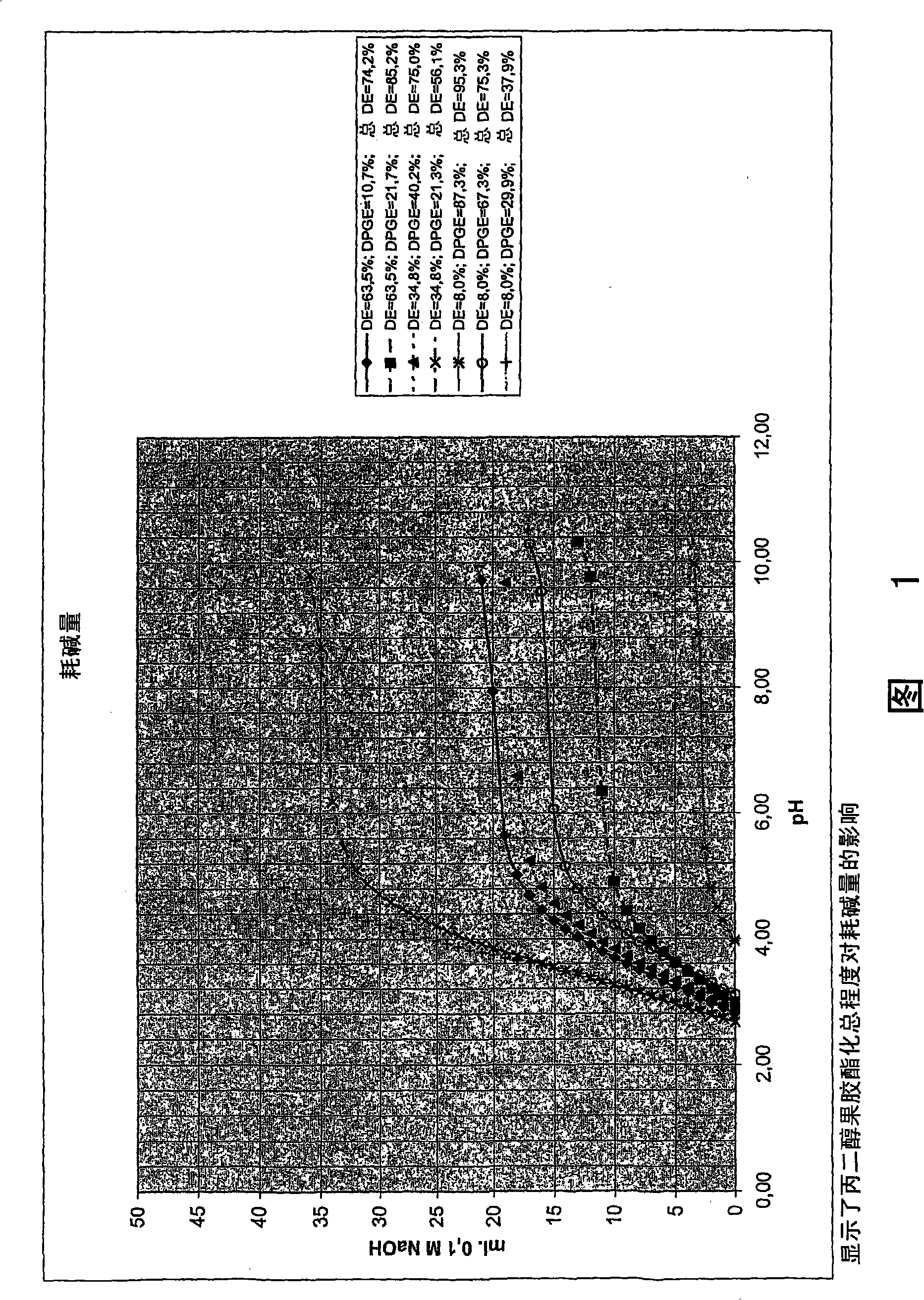
[0153] Figure 1 depicts the results shown in Table 2 above.
[0154] As can be seen from Figure 1, the alkali consumption (or buffer capacity) of propylene glycol pectin decreases with the total degree of esterification. This is consistent with the results for methylated pectin and propylene glycol alginate...
Embodiment 2
[0156] Example 2: Ability to Lower pH
[0157] The pH drop measurement was then used to assess the ability of the same 7 sample fractions to drop pH. The pH drop was detected by the following experimental protocol:
[0158] Procedure for Measuring pH Reduction
[0159] 1. Dissolve 1 g of pectin in 100 g of deionized water at a specific dissolution temperature.
[0160] 2. Place the solution in a thermostatically controlled water bath with constant stirring.
[0161] 3. Bring the pH between 9 and 10 by adding 0.1M NaOH.
[0162] 4. Record the pH as a function of time.
[0163] Table 3 below lists the assay results.
[0164] table 3
[0165]
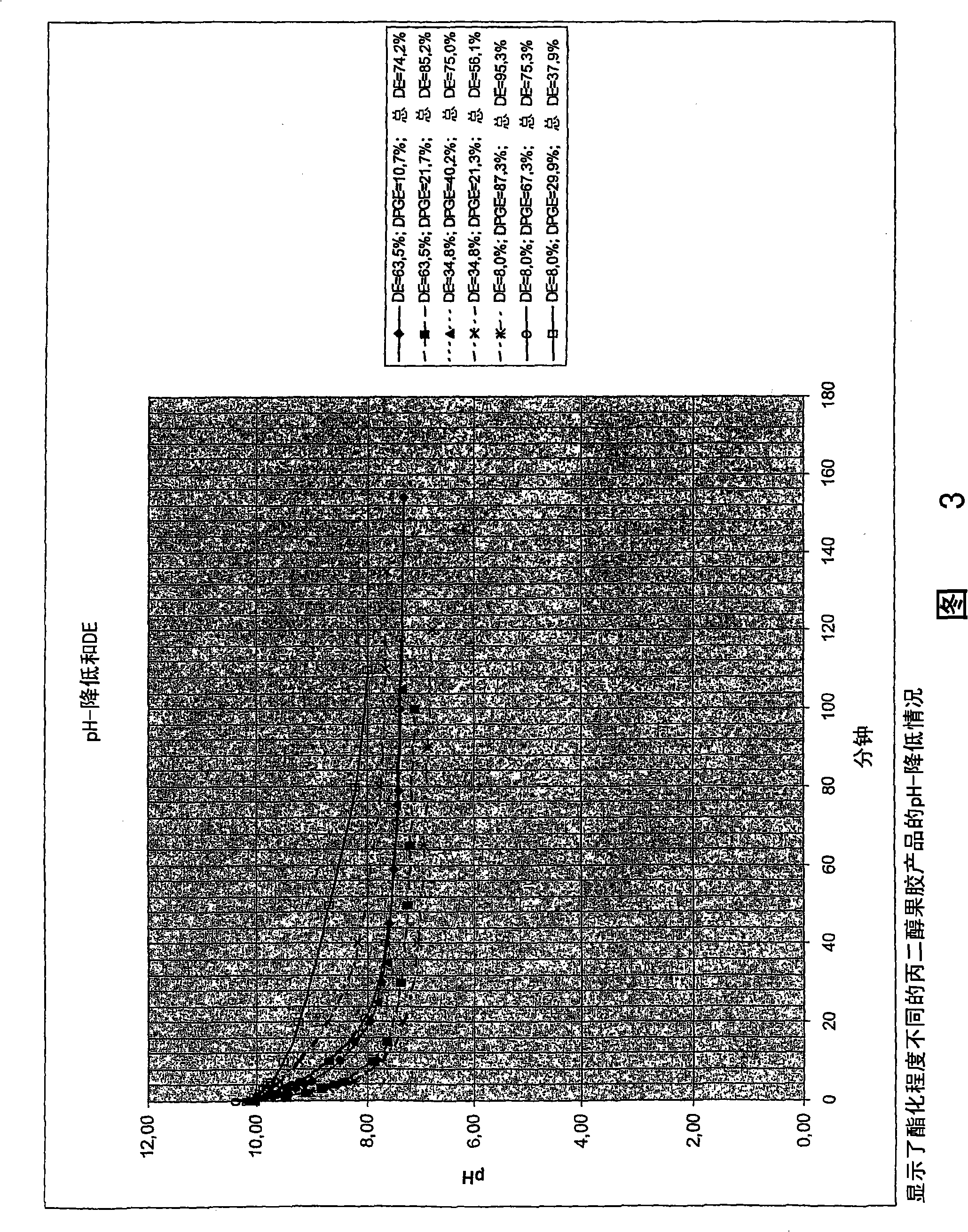
[0166] Figure 3 depicts the results shown in Table 3 above.
[0167] From Figure 3, it can be seen that the pH decrease increases with the total degree of esterification. Therefore, in this respect, the performance of propylene glycol pectin is similar to that of methylated pectin and propylene glycol alginate.
[0168] Figur...
Embodiment 3
[0169] Example 3: Effect of temperature
[0170] A sample of Reaction 6 was then studied to further determine the effect of temperature during pH reduction. The detection was carried out according to the above "Procedure for determining the pH-decrease", but the temperature was maintained in two different temperature ranges: the pH reduction in step (4) was carried out in two different temperature ranges of 30-32°C and 45-47°C. The results are shown in Table 4 below.
[0171] Table 4
[0172]
[0173] Figure 5 depicts the results shown in Table 4 above.
[0174] It can be seen from Figure 5 that for the two identical samples, the pH decreases faster at high temperature. Thus, similar to methylated pectin and propylene glycol alginate, propylene glycol glycerol pectin deesterifies faster at higher temperatures, resulting in a faster decrease in pH with increasing temperature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of esterification | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com