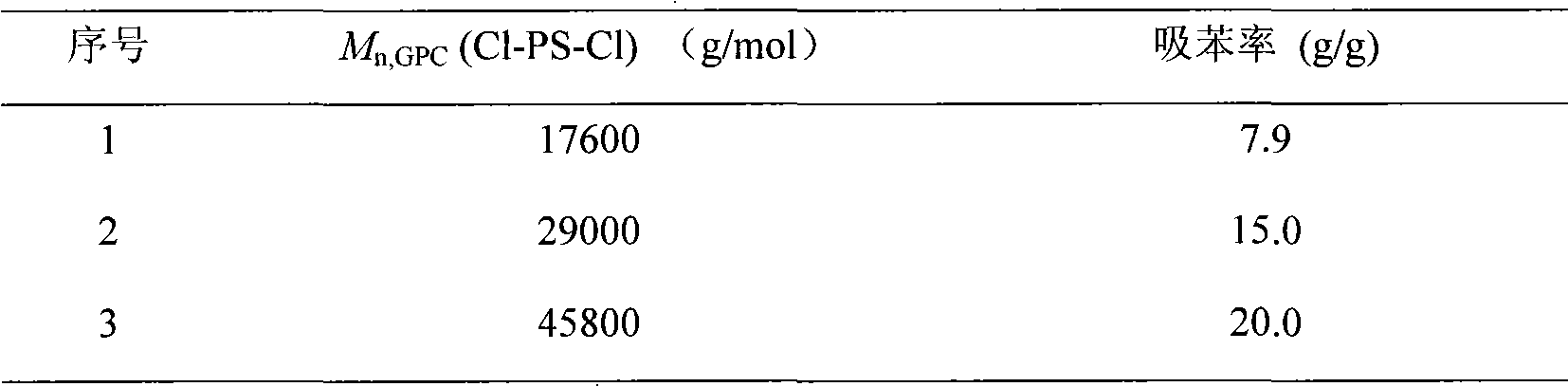Process for synthesizing pipetting block copolymer gel
A block copolymer and liquid-absorbing technology, which is applied in the field of synthesizing liquid-absorbing block copolymer gels, can solve the problems of atom transfer radical synthesis that have not been reported in the literature, and achieve uniform molecular weight distribution of chain segments, Convenient design and controllable crosslink density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of polystyrene (PS) bifunctional macromolecular initiator
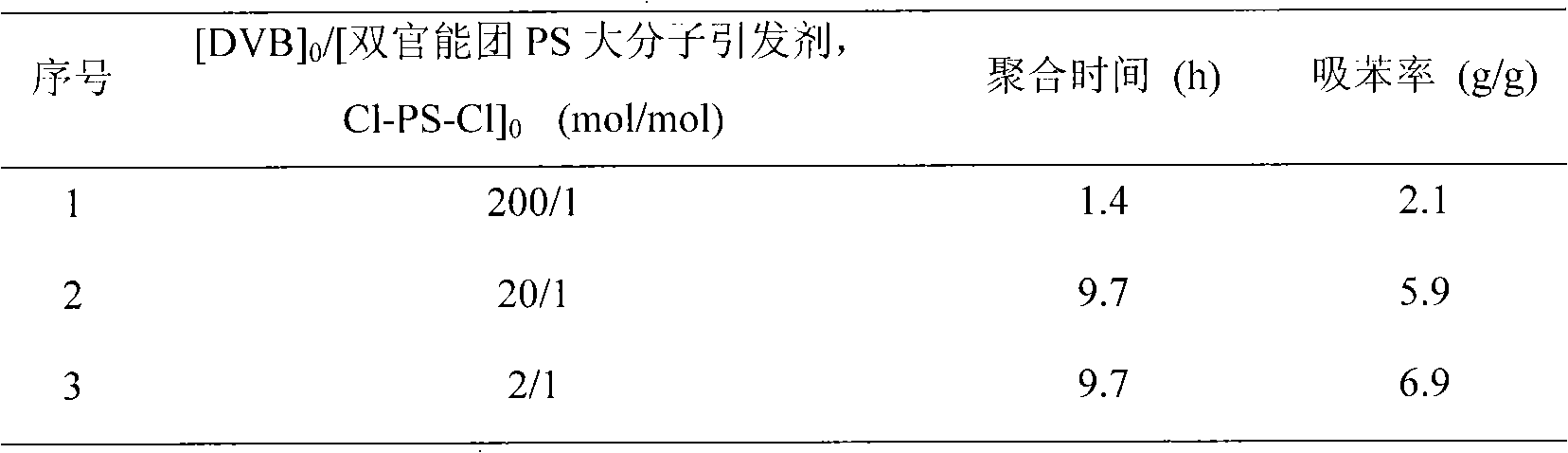
[0035] According to the ratio n(styrene):n(α,α'-dichloro-p-xylene (DCX)):n(CuBr):n(2,2'-bipyridine (bpy))=200~1000:1: 1:3, sequentially add CuBr, bpy, DCX and styrene to a 10mL ampoule, vacuum-fill with N 2 - Vacuum, repeat 5 times, seal the tube under vacuum, and place it in an oil bath at a constant temperature of 130° C. for a predetermined time (45 h) to react.
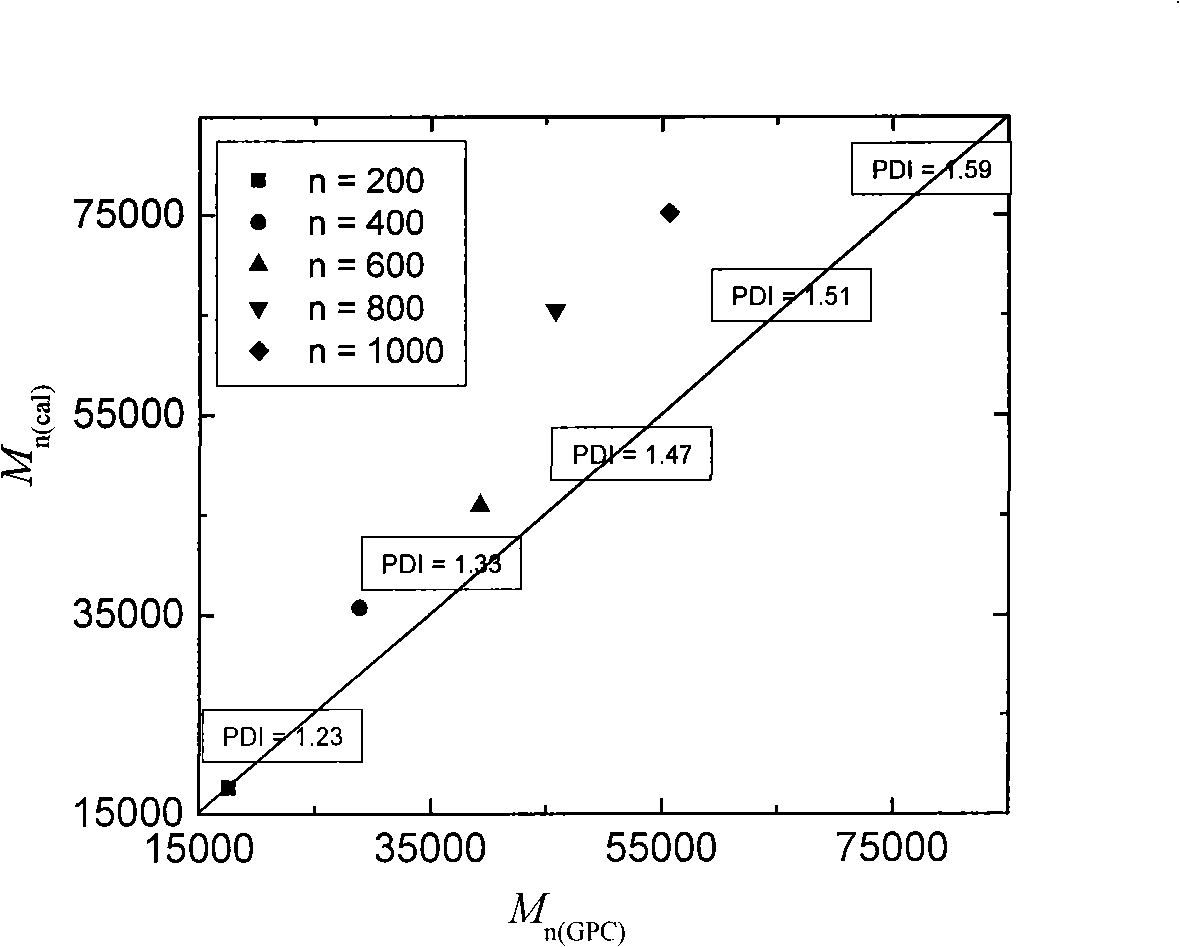
[0036] After the reaction, take out the sealed tube, cool it with cold water immediately, open the sealed tube, dissolve the polymer PS with THF, methanol is used as a precipitant, collect the polymer, dry it in a vacuum oven at 50°C to constant weight, and calculate the conversion rate. The relational curve of its theoretical molecular weight and measured molecular weight is as follows figure 1 As shown, it exhibits very good characteristics of living / controlled polymerization.
[0037] (2) Synthesis of PBA-b-PS-b-PBA triblock cop...
Embodiment 2
[0044] (1) Synthesis of polybutyl acrylate difunctional macroinitiator
[0045] According to the ratio n(butyl acrylate):n(α,α'-dichloro-p-xylene (DCX)):n(CuBr):n(2,2'-bipyridine (bpy))=200~1000:1 : 1: 3, sequentially add CuBr, bpy, DCX and butyl acrylate in a 10ml ampoule, vacuum-fill with N 2 - Vacuum, repeat 5 times, seal the tube under vacuum, and place it in an oil bath at a constant temperature of 130° C. to react for a predetermined time (137 h).
[0046] After the reaction, take out the sealed tube, cool it with cold water immediately, open the sealed tube, dissolve the polymer PS with THF, methanol is used as a precipitant, collect the polymer, dry it in a vacuum oven at 50°C to constant weight, and calculate the conversion rate.
[0047] (2) Synthesis of PS-b-PBA-b-PS triblock copolymer
[0048] Add PBA bifunctional macromolecular initiator (M n,GPC =10210g / mol, PDI=1.38; 0.57g, 0.057mmol), 2.0ml of toluene was shaken and mixed until the PBA was completely dissolv...
Embodiment 3
[0065] Example 3: Post-crosslinked amphiphilic three-arm diblock copolymer gel
[0066] (1) Synthesis of trifunctional polymethyl methacrylate (PMMA) macroinitiator
[0067] According to the ratio n(MMA):n(BTBiB):n(CuCl):n(bpy)=100~500:1:1:3, add CuCl, bpy, DMF, BBTiB and MMA in 5mL ampoule in turn, Vacuum-N filled 2 -Vacuum, repeat 5 times, seal the tube under vacuum, and place it in an oil bath at a constant temperature (90° C.) for a predetermined time (18 h) to react.
[0068] After the reaction is over, take out the sealed tube, cool it with cold water immediately, open the sealed tube, dissolve the polymer (PMMA) with THF, use methanol as a precipitant, collect the polymer, and dry it in a vacuum oven at 50°C to constant weight to obtain the product PMMA .
[0069] (2) Synthesis of star amphiphilic PMMA-b-PDMAEMA three-arm macroinitiator
[0070] According to the proportioning n(DMAEMA):n(PMMA):n(CuCl):n(bpy)=300~800:1:1:3, add CuCl, bpy, DMF, PMMA and DMAEMA in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com