Blue-light emitting functional material and uses thereof
A functional material, blue light technology, applied in the field of oligomer materials and its application in the field of optoelectronic materials, the field of oligomer functional materials containing tricarbazole units, can solve the problem of unfavorable charge injection and transport, phase separation photoelectric Loss and other issues, to achieve the effect of improving luminous brightness, inhibiting crystallinity, and improving blue light emission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
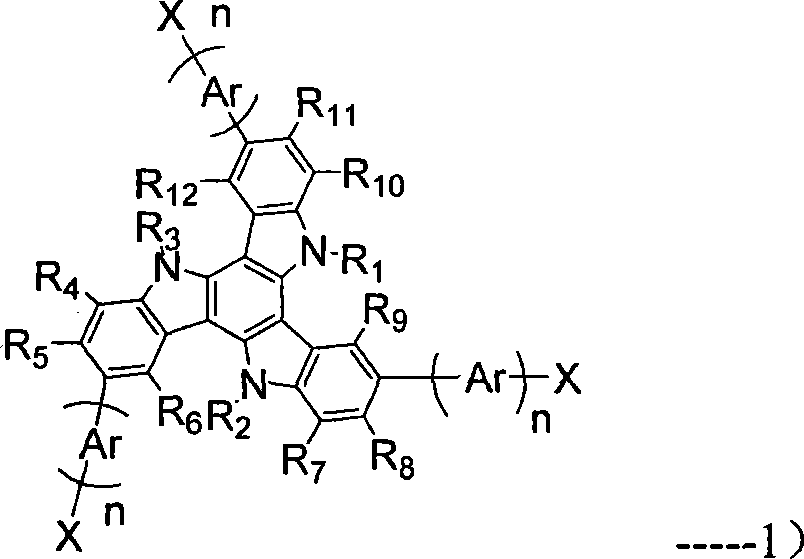
[0053] The preparation of the target product includes three parts, the first is the preparation of the multifunctional tricarbazole core; the second is the preparation of the monofunctional oligomer branch; the last is the preparation of the star-shaped target product.
[0054] Specifically, firstly, the trifunctional tricarbazole compound 3 was prepared according to the reaction scheme 1.
[0055] 【Reaction Scheme 1】
[0056]
[0057] In the formula, R 1 , R 2 and R 3 Each is independently hydrogen, substituted or unsubstituted C1-C12 alkyl, substituted or unsubstituted C1-C12 alkoxy. Compound 1 is an alkylated tricarbazole derivative, which can be dehalogenated under the action of Pd / C catalyst to obtain Compound 2. The tribrominated tricarbazole derivative 3 was obtained by selective bromination.
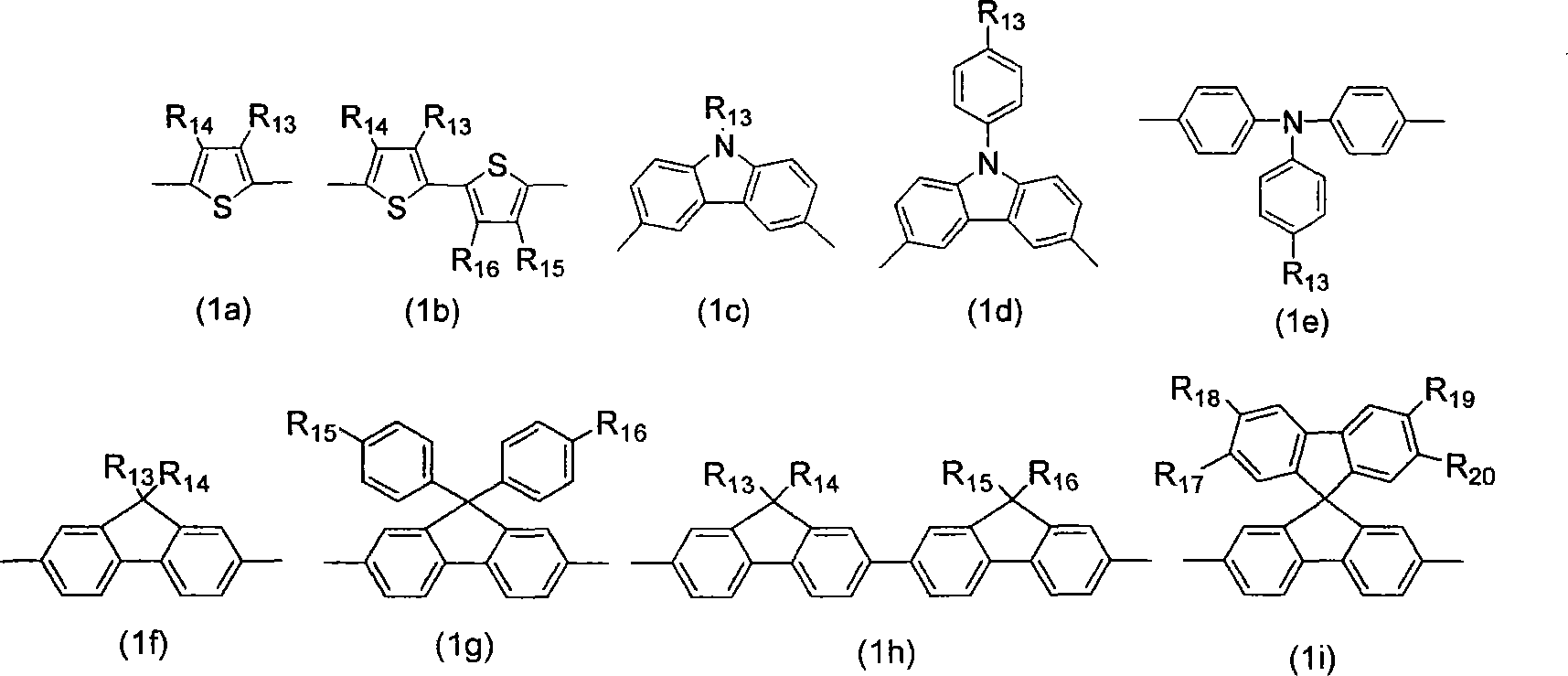
[0058] Then, according to the reaction scheme 2, monofunctionalized oligomeric fluorene monomers 5, 7, 9 and 11 with well-defined structures and chain lengths were prepar...
Embodiment 1
[0103] Example 1: Synthesis of 2,3,7,8,12,13-hexabromo-5,10,15-trihexyltricarbazole
[0104] 2,3,7,8,12,13-hexabromo-tricarbazole (2.45g, 3mmol) and KOH (1.4g, 25mmol) were dissolved in THF (100ml), heated to reflux, 1-bromohexane ( 2.23g, 13.5mmol) was added dropwise to the above solution, and refluxed for 3h. The mixture was cooled to room temperature and washed with CH 2 Cl 2 Diluted, then washed the organic layer with 10% HCl solution, saturated NaCl solution, and finally with anhydrous NaCl 2 SO 4 dry. The crude product obtained after removal of the organic solvent was isolated by column chromatography to give a white solid (2.96 g, 92%).
Embodiment 2
[0105] Embodiment 2: the synthesis of 5,10,15-trihexyl tricarbazole
[0106] 2,3,7,8,12,13-hexabromo-5,10,15-trihexyltricarbazole (2.40g, 2.24mmol), triethylamine (2.5ml), formic acid (0.68ml) and 10 A THF solution (20 mL) of %Pd / C (712.3 mg, 0.67 mmol) was heated to reflux for 3 hours. The mixture was filtered, the filtrate was diluted with chloroform, washed with 10% aqueous hydrochloric acid, washed with anhydrous Na 2 SO 4 The organic layer was dried and finally the solvent was removed. The resulting crude product was separated by column chromatography to obtain a white solid powder (1.25 g, 93%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com