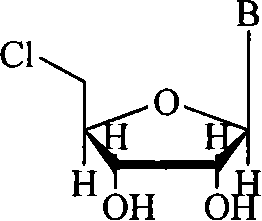
Method for synthesizing 5'-chloro-nucleoside
A technology for chlorinated nucleosides and synthesis methods, applied in the directions of sugar derivatives, organic chemistry, etc., can solve the problems of high cost, complicated processing, low yield, etc., and achieves the development and application, mild reaction conditions and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 uses adenosine as raw material to prepare 5'-chlorinated nucleosides
[0017] 1g of adenosine (3.7mmol) and 3.33g of triphenylphosphine (12.7mmol) were dissolved in 25ml of dichloromethane, protected by argon, and 3.01g of hexachloroethane (12.7mmol) was added dropwise under reflux. After stirring under reflux at 40°C for 10 hours, cool to room temperature at 15°C, add acetic acid corresponding to 50% of the volume of the reaction solution, stir at 70°C for 3 hours, and evaporate the solvent to dryness under reduced pressure. Wash with dichloromethane until TLC shows that the washing liquid no longer contains triphenoxyphosphine. After column chromatography using 3% methanol / dichloromethane as eluent, white powdery crystals are obtained with a yield of 83.8%.
[0018] 1 H NMR ((CD 3 ) 2 SO, 400MHz): δ8.36(s, 1H, 2-H), 8.16(s, 1H, 8-H), 7.32(s, 1H, -NH 2 ), 5.93 (d, J=5.6Hz, 1H, 1′-H), 5.62 (d, J=6.0Hz, 1H, 2′-OH), 5.45 (d, J=5.4Hz, 1H, 3′- OH), 4.75(m,...
Embodiment 2
[0019] Example 2 Preparation of 5'-chloronucleosides with guanosine as raw material
[0020] 1 g of guanosine (3.5 mmol) and 3.24 g of triphenylphosphine (12.3 mmol) were dissolved in 25 ml of dichloromethane, protected by argon, and 2.91 g of hexachloroethane (12.3 mmol) was added dropwise under reflux. After stirring under reflux at 50°C for 10 hours, cool to room temperature 25°C, add acetic acid corresponding to 50% of the volume of the reaction solution, stir at 70°C for 3 hours, and evaporate the solvent to dryness under reduced pressure. Wash with dichloromethane until TLC shows that the washing liquid no longer contains triphenoxyphosphine. After column chromatography using 15% methanol / dichloromethane as eluent, white powdery crystals are obtained with a yield of 86.1%.
[0021] 1 H NMR (D 2 O, 400MHz) δ7.93(s, 1H, 8-H), 5.80(d, J=4.8Hz, 1H, 1'-H), 4.70(m, 1H, 2'-H), 4.37(m, 1H, 3′-H), 4.28 (m, 1H, 4′-H), 3.80 (m, 2H, 5′-2H); ESIMS (C 10 h 12 ClN 5 o 4 ): 302.1...
Embodiment 3
[0022] Example 3 Preparation of 5'-chloronucleosides with cytidine as raw material
[0023] 1 g of cytidine (4.1 mmol) and 3.78 g of triphenylphosphine (14.4 mmol) were dissolved in 25 ml of cyclohexane, protected by argon, and 3.41 g of hexachloroethane (14.4 mmol) was added dropwise under reflux. After stirring under reflux at 30°C for 10 hours, cool to room temperature at 20°C, add 50% acetic acid corresponding to the volume of the reaction solution, stir at 70°C for 3 hours, and evaporate the solvent to dryness under reduced pressure. Wash with dichloromethane until TLC shows that the washing liquid no longer contains triphenoxyphosphine. After column chromatography using 15% methanol / dichloromethane as eluent, white powdery crystals are obtained with a yield of 89.6%.
[0024] 1 H NMR ((CD 3 ) 2 SO, 400MHz): δ7.60 (d, J=7.4Hz, 1H, 6-H), 7.26 (br, 2H, -NH 2 ), 5.82 (d, J=4.6Hz, 1H, 1′-H), 5.75 (d, J=7.4Hz, 1H, 5-H), 5.43 (d, J=5.6Hz, 1H, 2′-OH ), 5.30(d, J=5.6Hz, 1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com