Kitasamycin microcapsule preparation and preparation and application thereof
A kitasarmycin preparation technology, applied in the field of preparation of antibacterial and growth-promoting agents for livestock and poultry, can solve the problems of poor antibacterial and growth-promoting effects, need to contact organic solvents, long coating process time, etc., to achieve the advantages of mixing, Good fluidity, avoiding hepatic first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
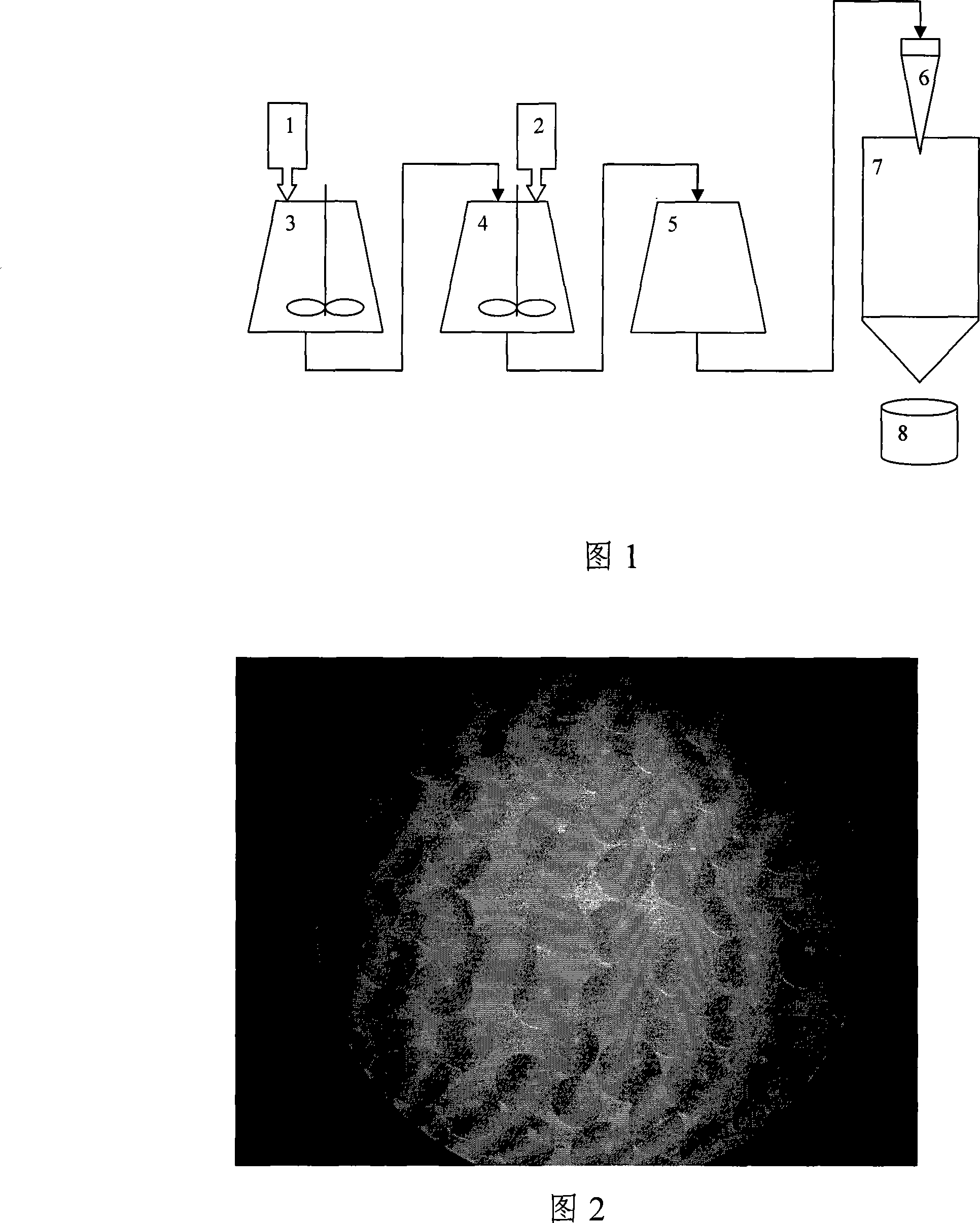
[0031] Referring to accompanying drawing 1, select auxiliary material 1 stearic acid 225kg to drop into material tank 3, be heated to 55-120 ℃, keep stirring, until auxiliary material 1 is all melted; Melted stearic acid is pumped into batching tank 4 from chemical material tank, Simultaneously, 100 kg of kitasamycin (kitasamycin raw material 2) of titer 1636 μ / mg that has been accurately weighed is dropped into batching tank 4, high-speed emulsification shearing and stirring for 5 minutes, temperature will be controlled at 80 ℃-100 during the meantime; The good material is introduced into the storage tank 5, and heated to 55-120°C; the material delivery spray device 6 is started, and the material in the storage tank 5 is sprayed and granulated, and the material delivery speed is controlled at 0.5-0.8m 3 / min, atomizing disk rotation speed 4000~6000n / min, when the ambient temperature is above 30°C, control the inlet air temperature ring at 10~12°C, when the ambient temperature ...
Embodiment 2
[0034] Select auxiliary material 1 stearic acid 368kg into chemical material tank 3, heat to 80-100°C, stir continuously, until the auxiliary material is completely melted; pump the melted stearic acid from chemical material tank into compounding tank 4, and accurately weigh 25kg of kitasamycin (raw material 2) of kitasamycin with a titer of 1582μ / mg is put into the batching tank 4, high-speed emulsification, shearing and stirring for 60 minutes, during which the temperature will be controlled at 80°C-100°C; Material tank 5, and heat the temperature to 80°C-100°C; start the conveying spray device 6, spray and granulate the material in the material storage tank 5, and control the material conveying speed to 0.5-0.8m 3 / min, atomizing disk rotation speed 4000~6000n / min, when the ambient temperature is above 30°C, control the inlet air temperature ring at 10~12°C, when the ambient temperature is 20~29°C, control the inlet air temperature at 15~22°C, and when the ambient temperatur...
example 3
[0037] 400kg of auxiliary material 1 stearic acid is selected and put into chemical tank 3, heated to 80°C, and stirred continuously, until the auxiliary material is completely melted; Put 50kg of kitamycin (raw material 2) of kitamaycin with a price of 1617μ / mg into batching tank 4, high-speed emulsification, shearing and stirring for 12 minutes, during which the temperature should be controlled at 80°C-100°C; the prepared materials are imported into the storage tank 5, and heat the temperature to 80°C-100°C; start the conveying spray device 6, and spray and granulate the material in the storage tank 5, wherein the material conveying speed is controlled at 0.5-0.8m 3 / min, atomizing disk rotation speed 4000~6000n / min, when the ambient temperature is above 30°C, control the inlet air temperature ring at 10~12°C, when the ambient temperature is 20~29°C, control the inlet air temperature at 15~22°C, and when the ambient temperature is below 19°C Control the inlet air temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com