Use of 3h-1,2-dithiole-3-thione, anethole dithiolethione, sulphoraphane, phenethyl isothiocyanate, 6-(methylsulphinyl)hexyl isothiocyanate and allyl isothiocyanate for the treatment of canities
A technology of heterocyclopentadienethione and thiolene, applied in the application field of several dithiolanthione and isothiocyanate compounds in the treatment of gray hair , capable of addressing the lack of identification of the cause of specific and progressive depletion of melanocytes and melanocyte precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 - Determination of the activity of useful compounds of the present invention
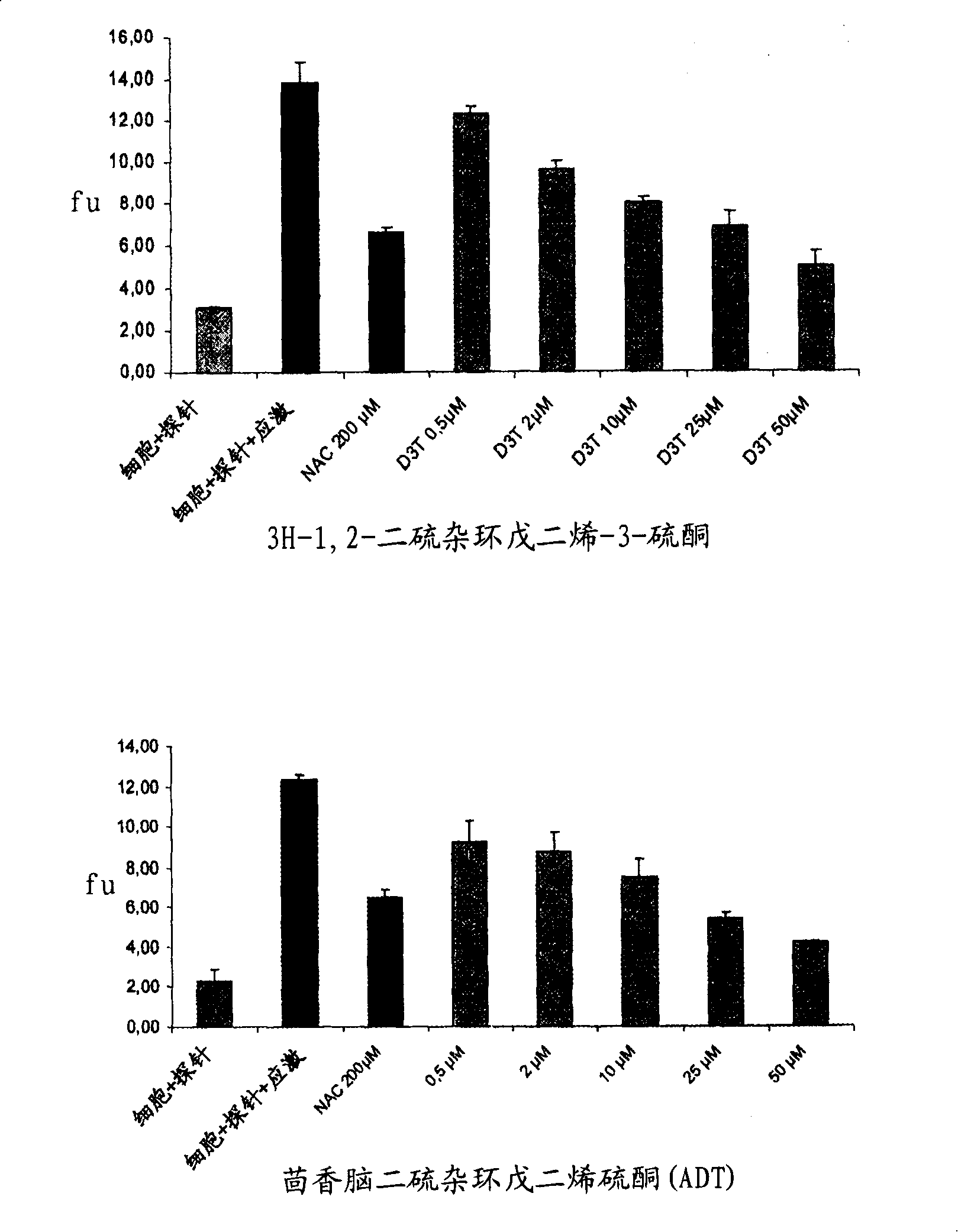
[0084] 1-A-1. Determination of the passage of H in cultured normal human melanocytes (NHM) 2 O 2 stress-induced activity Oxygen species (ROS) scheme
[0085] Normal human melanocytes (NHMs) at a density of 4×10 4 cells / cm 2 inoculated on the DO. In D1, the medium was replaced with 10 μM 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, bis[methyl acetate] in PBS (H 2 DCFDA, C2938, Molecular Probes). 20 minutes later, H 2 DCFDA solution was replaced with culture medium. After joining H 2 o 2 The NHMs were allowed to stand for 30 min before solution (250 μM). Fluorescence was measured after 15 minutes in a fluoroscope (activation: 485 nm, emission: 538 nm).
[0086] The use of research compounds depends on their nature (concentration, pre-incubation time). N-acetylcysteine (A9165-Sigma) was used as a control molecule. At 37°C, melanocytes were pretreate...
Embodiment 2
[0098] Example 2 - Composition
[0099] -shampoo
[0100] 3H-1,2-Dithiole-3-thione 0.5g
[0101] Propylene Glycol 20g
[0102] 95° ethanol 30g
[0103] Appropriate amount of water to 100g
[0104] The shampoo is used daily on the treated area, preferably on the entire scalp for at least 10 days, preferably for 1 to 2 months. A reduction in the appearance of white or gray hair followed by recoloration of the gray hair is observed.
[0105] treatment shampoo
[0107] Polyglyceryl-3 hydroxyaryl ether 26g
[0108] Hydroxypropylcellulose called KLUCELL G by Hercules 2g
[0109] Appropriate amount of preservatives
[0110] 95° ethanol 50g
[0111] Appropriate amount of water to 100g
[0112] The shampoo was used in each wash with a contact time of about 1 minute. Prolonged use for about two months resulted in a reduction in gray hair and a gradual recoloration of the gray hair.
[0113] This shampoo can also be used prophylactically t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com