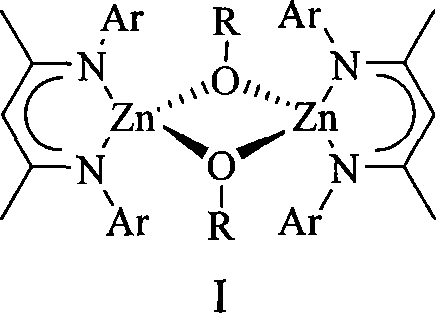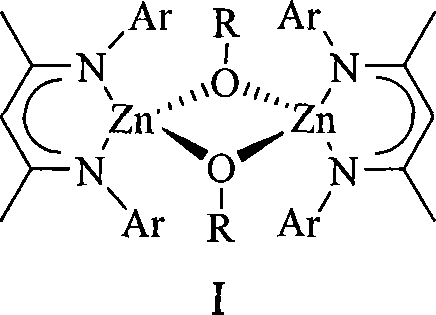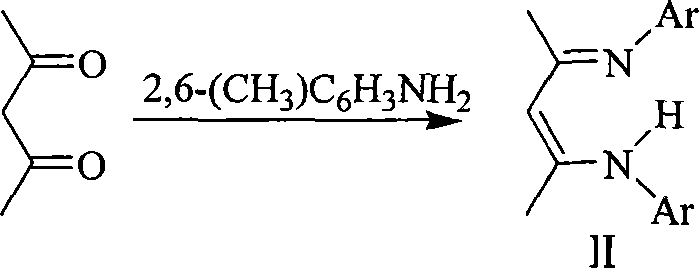Alkoxyl compound of beta-ketone di-imidogen zincium, preparation and application thereof
A ketodiimide zinc and alkoxy compound technology is applied in the field of element organic compound synthesis and catalysis, can solve the problems of difficult preparation and high synthesis cost, and achieves the effects of simple preparation method, high catalytic activity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of β-ketodiimine II: Take 100g of 2,6-dimethylaniline (>99%), 41.3g of 2,4-pentanedione, and 35mL of concentrated hydrochloric acid into a 500mL three-necked flask, add methanol to dissolve and reflux After 48 hours, filter, add NaOH solution to the obtained filtrate to adjust the pH>7.2, then use CH 2 Cl 2 (50mL×3) extraction, the organic phases were combined and dried with anhydrous magnesium sulfate to remove CH 2 Cl 2 The obtained solid was recrystallized from n-hexane to obtain 66.0 g of colorless massive crystal II with a yield of 53%. mp 77-79°C.
[0029] 2) Preparation of the ethyl compound III of β-ketodiimine zinc: take 6.13g (20mmol) of β-ketodiimine compound II into a 100mL Schlenk bottle, and vacuumize the oil pump for 1 hour. Add 40mL of toluene to dissolve the compound, add 30mmol ZnEt 2 The toluene solution was stirred at room temperature for 1 hour, heated to reflux and continued to react for 3 hours, the volatile substances were rem...
Embodiment 2
[0034] β-ketodiimino zinc alkoxylate I b Preparation: Add 0.37g (0.50mmol) of the β-ketodiimino zinc hydrogen bridge compound V obtained in step 4) of Example 1 to a 100mL Schlenk bottle, dissolve it in 20mL of toluene, and add equimolar Benzaldehyde toluene solution 2M (0.50mL, 1.0mmol), after 4 hours of reaction, a white precipitate was obtained, which crystallized at room temperature after heating and dissolving to obtain 0.38g of β-ketodiiminozinc alkoxy compound I b , yield 80%. 1 H NMR (C 6 D. 6 , 400MHz): 7.31 [d, 4H, CH 2 C 6 h 5 (m-H)], d 7.13 (m, 12H, ArH), 7.12 [m, 4H, CH 2 C 6 h 5 (p-H)], 7.07 [s, 4H, CH 2 C 6 h 5 (o-H)], 4.92 (s, 2H, γ-CH), 4.85 [s, 4H, Zn-(μ-OCH 2 C 6 h 5 )], 1.96(s, 24H, Ar-CH 3 ), 1.50(s, 12H, β-CCH 3 ).
Embodiment 3
[0036] β-ketodiimino zinc alkoxylate I b Preparation: Add 0.37 g (0.50 mmol) of the β-ketodiimino zinc hydrogen bridge compound V obtained in Step 4) of Example 1 to a 100 mL Schlenk bottle, dissolve it in 20 mL of toluene, add it at -75 ° C, etc. Mole of benzyl alcohol toluene solution 2M (0.50mL, 1.0mmol), the reaction temperature was gradually raised to room temperature and continued to react for 4h, a white precipitate was obtained, which crystallized at room temperature after heating and dissolving to obtain 0.32g β-ketodiiminozinc alkoxide base compound I b , yield 67%. 1 H NMR (C 6 D. 6 , 600MHz): 7.32 [d, 4H, CH 2 C 6 h 5 (m-H)], d 7.13 (m, 12H, ArH), 7.12 [m, 4H, CH 2 C 6 h 5 (p-H)], 7.07 [s, 4H, CH 2 C 6 h 5 (o-H)], 4.92 (s, 2H, γ-CH), 4.85 [s, 4H, Zn-(μ-OCH 2 C 6 h 5 )], 1.96(s, 24H, Ar-CH 3 ), 1.50(s, 12H, β-CCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com