Compound with substituted cyclopropane structure, production method and medicine use thereof
A kind of cyclopropane and compound technology, applied in the field of a class of compounds with substituted cyclopropane structure, preparation and medical use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
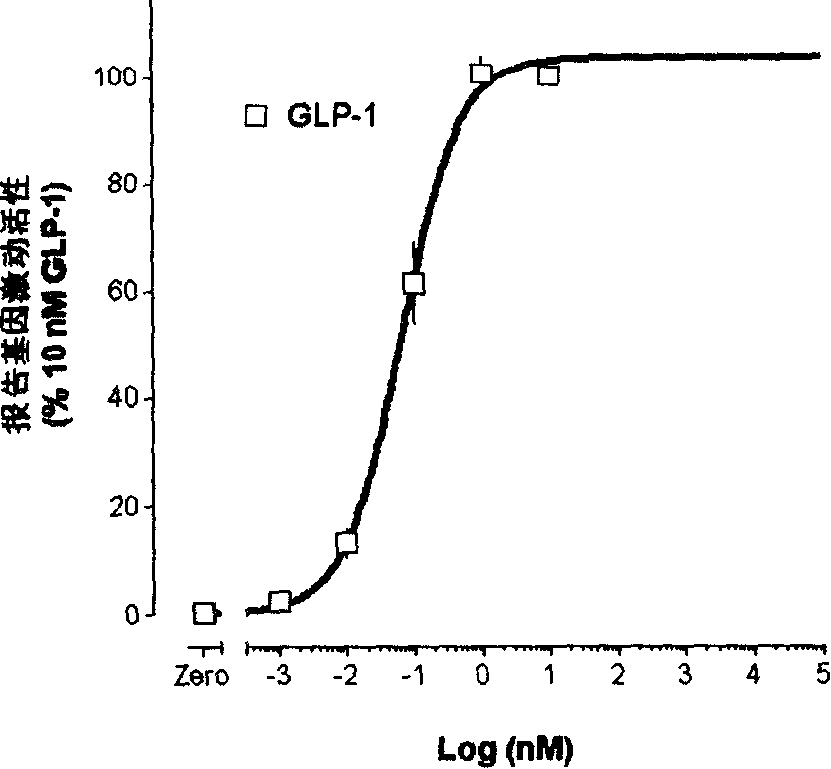
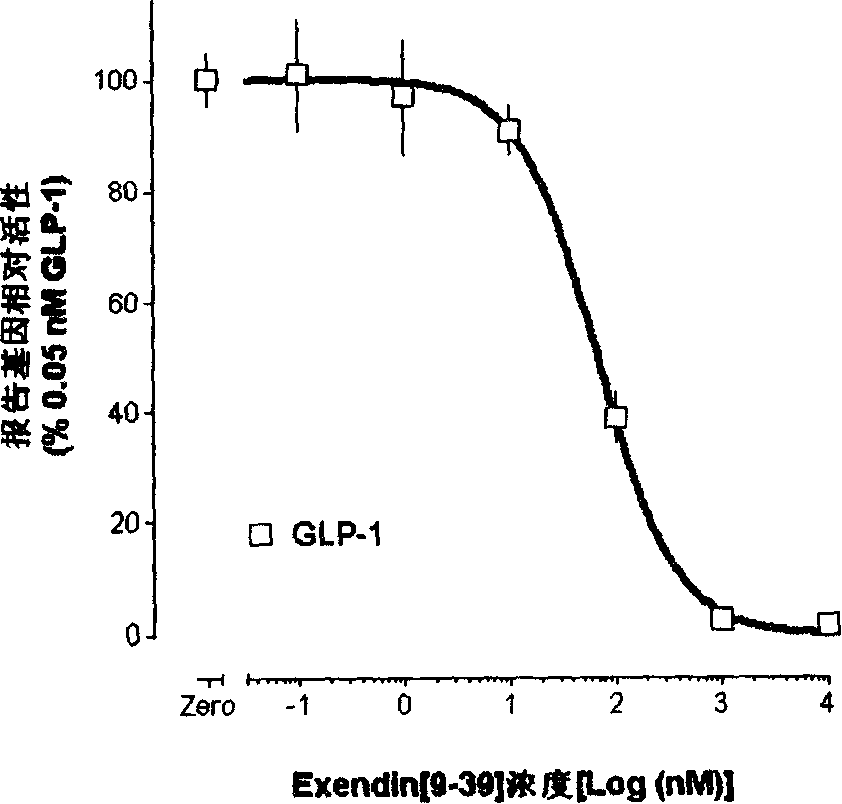
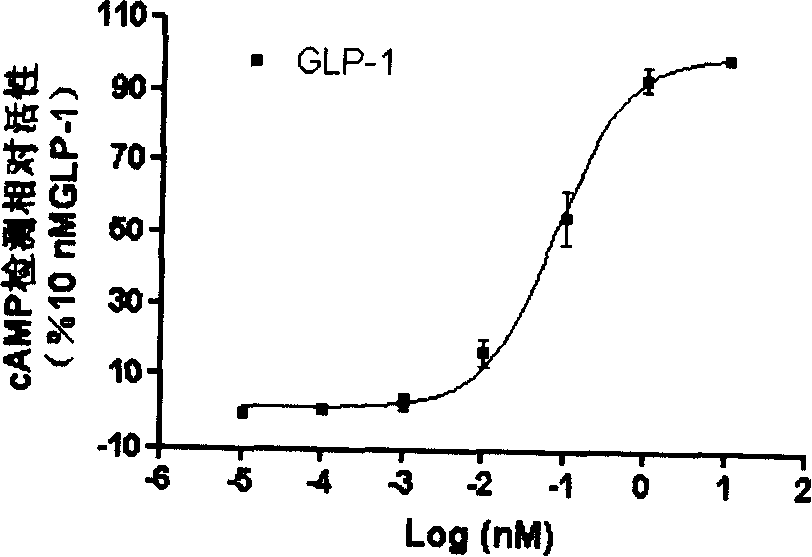
Image
Examples
Embodiment 1
[0162] NMR calibration: δH / C 7.26 / 77.0ppm (CDCl3); δH / C 2.50 / 39.51ppm (DMSO-d6).
[0163]
[0164]Compound 2,3-diaminosuccinic acid was dissolved in an appropriate amount of 5% sodium hydroxide solution, cooled to 0°C, p-BocNH benzoyl chloride (2.5eq) was added dropwise, left at room temperature overnight, acidified to pH=2 with hydrochloric acid, Extracted with ether and concentrated to dryness. Recrystallization from ethanol / water afforded the amide.
[0165] The above amide (1eq) was dissolved in methanol, a few drops of concentrated sulfuric acid were added dropwise, and heated to reflux for 8 hours to obtain the ester.
[0166] The above ester and thionyl chloride were heated and reacted for 2 hours to obtain a ring compound.
[0167] The above compound (2eq) was heated to 180°C under the protection of nitrogen, and phenyldiazomethane (1eq) was slowly added dropwise at a rate to maintain the reaction temperature at 180°C-190°C. Cooling gave an orange residue. Add a...
Embodiment 2
[0173]
[0174] Dissolve the raw material acid (1eq) in methanol, add a few drops of concentrated sulfuric acid, and heat to reflux for 8 hours to obtain the ester.
[0175] The above ester (2eq) was heated to 180°C under the protection of nitrogen, and the compound phenyldiazomethane (1eq) was slowly added dropwise at a rate to maintain the reaction temperature at 180°C-190°C. Cooling gave an orange residue. An appropriate amount of 10% ether / n-pentane was added and filtered to obtain a compound with a substituted cyclopropane structure.
[0176] Dissolve the above-mentioned compound having a substituted cyclopropane structure in methanol, add sodium hydroxide solution, heat and hydrolyze to obtain a product.
[0177] The same method obtains the following products:
[0178]
[0179]
Embodiment 3
[0181]
[0182] Dissolve the raw material acid (1eq) in methanol, add a few drops of concentrated sulfuric acid, and heat to reflux for 8 hours to obtain the ester.
[0183] The above ester (2eq) was heated to 180°C under the protection of nitrogen, and the compound phenyldiazomethane (1eq) was slowly added dropwise at a rate to maintain the reaction temperature at 180°C-190°C. Cooling gave an orange residue. An appropriate amount of 10% ether / n-pentane was added and filtered to obtain a compound with a substituted cyclopropane structure.
[0184]
[0185]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com