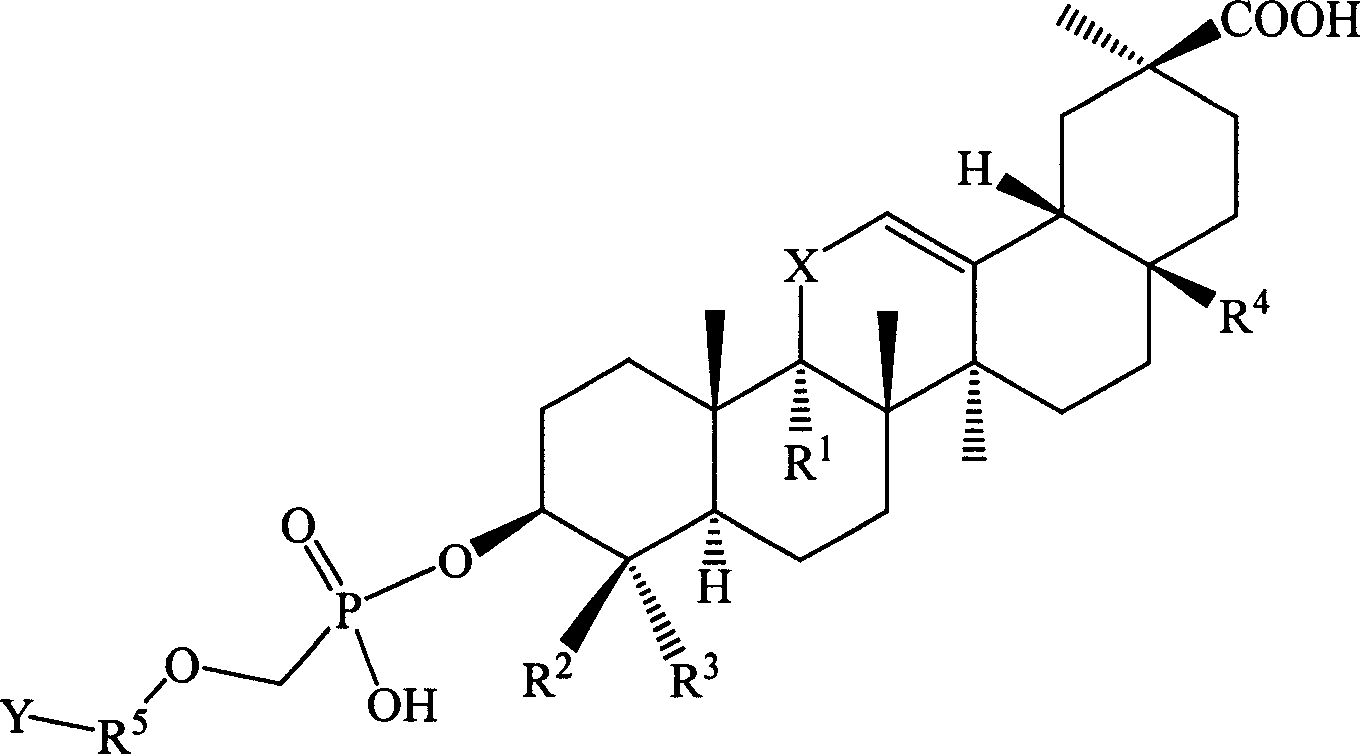
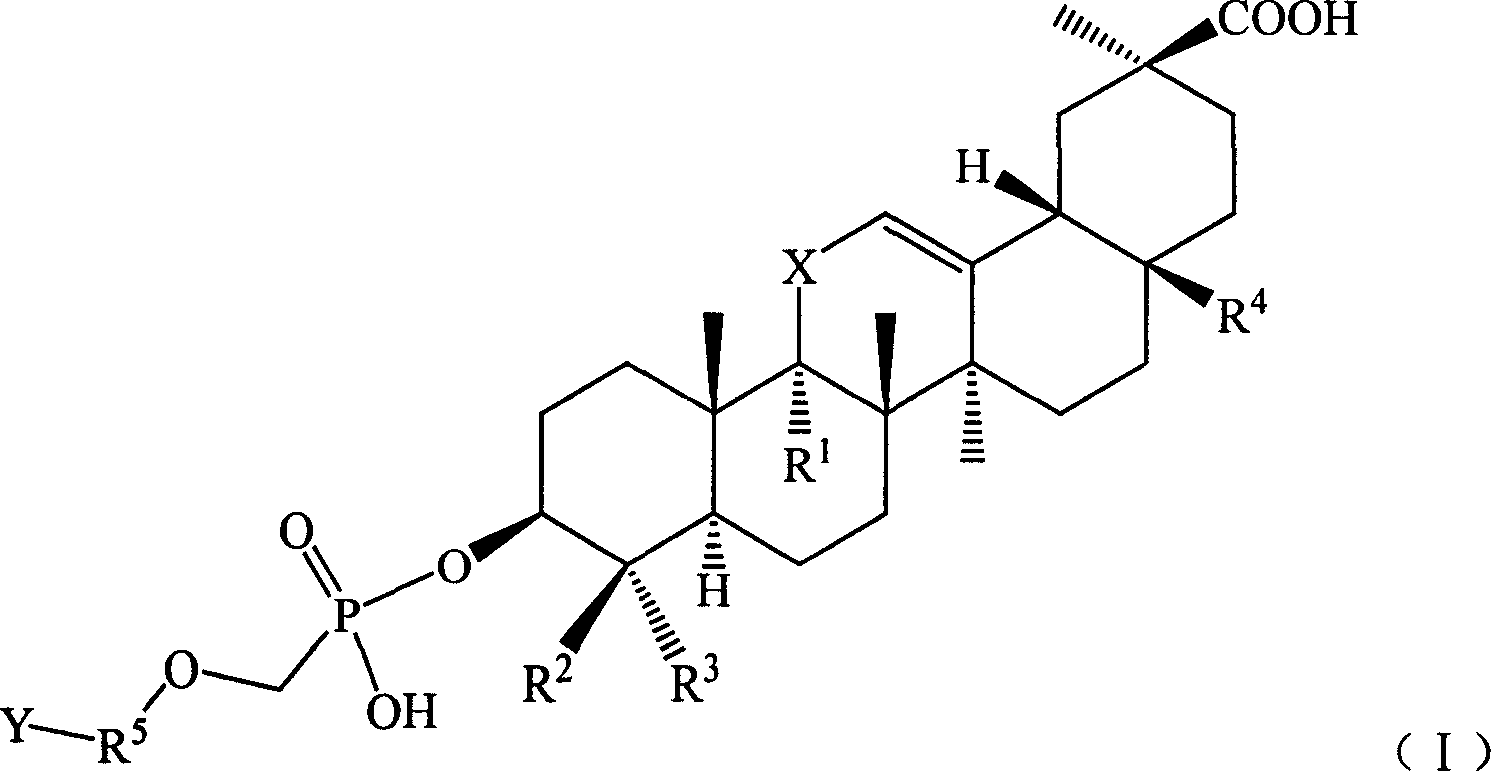
Compound used for viral infect
A compound and stereoisomer technology, applied in steroids, antiviral agents, digestive system, etc., can solve problems such as abnormal liver function, liver tissue damage, and difficulty in clearing viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1 Hydroxy-11′-oxo-18′β, 20′β-oleanane-12′-enoic acid-3′β-O-[2-[(adenine)-9-yl]B Oxygen]- Preparation of Methyl Phosphate Monoester
[0130] Suspend 27.3 g (0.1 mol) of 9-(phosphonomethoxy-ethyl) adenine in 100 mL of DMF, add 50 g of triethylamine, raise the temperature to 50°C and slowly add 3β-hydroxyl-11-oxygen in batches while stirring Dai-18β, 20β-oleanane-12-enoic acid 47.1g (0.1mol), stirred for 12h, the reaction solution was cooled, added 300ml of deionized water, after stirring, a solid precipitated, filtered, and the filter cake was washed with 200ml of ethyl acetate After dissolving, transfer it to a separatory funnel, wash with 20ml×3 water, then extract the organic layer with 1mol / l HCl50ml×3, combine the acidic water layer, and wash with saturated Na 2 CO 3 After adjusting the pH of the solution to 6, extract with 60ml × 3 ethyl acetate, combine the ethyl acetate layers and wash with saturated sodium chloride solution, dry the organic layer, f...
Embodiment 2
[0132] Example 2 11'-oxo-18'β, 20'β-oleanane-12'-enoic acid-3'β-O-[2-[(adenin)-9-yl]propoxy ]-Methylphosphonium Preparation of Acid Monoesters
[0133] Referring to the preparation method in Example 1, 9-(2-phosphonomethoxy-ethyl)adenine was replaced by 9-(2-phosphonomethoxy-propyl)adenine to obtain 11′-oxo Generation-18′β,20′β-Oleanane-12′-enoic acid-3′β-O-[2-[(Adenin)-9-yl]propoxy]-methylphosphonic acid monoester 34.9 g, yield: 47.1%.
[0134] Elemental analysis (C 39 h 58 N 5 o 7 P): C: 63.19%, H: 7.98%, N: 9.41%, P: 4.12% (theoretical: C: 63.31%, H: 7.90%, N: 9.47%, P: 4.19%).
Embodiment 3
[0135] Example 3 18'β, 20'β-Oleanane-12'-enoic acid-3'β-O-[2-[(Adenin)-9-yl]ethoxy]-methylphosphonic acid mono ester preparation of
[0136] Referring to the preparation method in Example 1, replace 3β-hydroxy-11-oxo-18β, 20β-oleanane-12-enoic acid with 3β-hydroxy-18β, 20β-oleanane-12-ene Acid to give 18′β, 20′β-oleanane-12′-enoic acid-3′β-O-[2-[(adenin)-9-yl]ethoxy]-methylphosphonic acid mono 32.2 g of ester, yield: 45.4%.
[0137] Elemental analysis (C 38 h 58 N 5 o 6 P): C: 64.02%, H: 8.28%, N: 9.81%, P: 4.30% (theoretical: C: 64.11%, H: 8.21%, N: 9.84%, P: 4.35%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com