Improved polymerases
A polymerase and amino acid technology, applied in the field of polymerase, can solve research problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
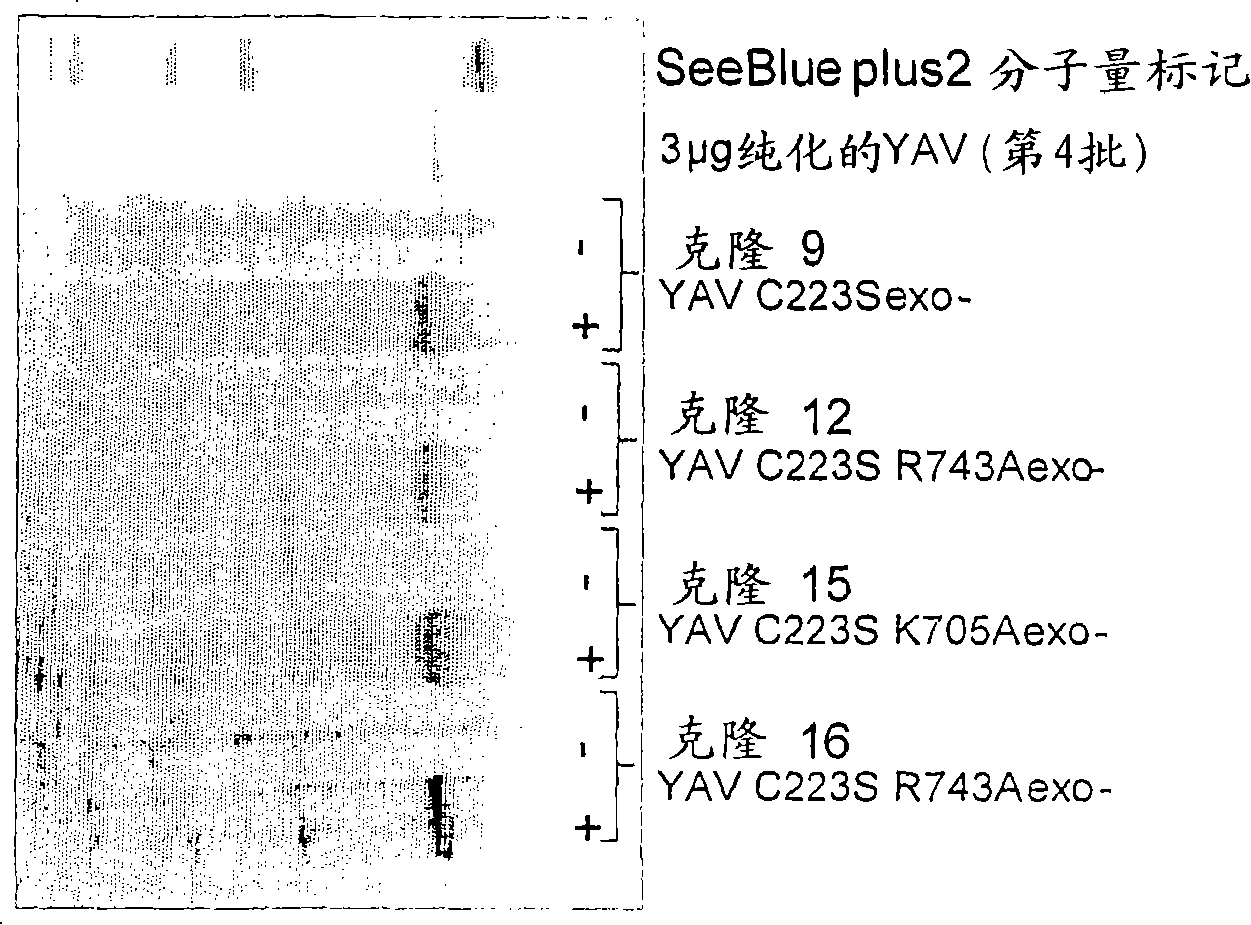
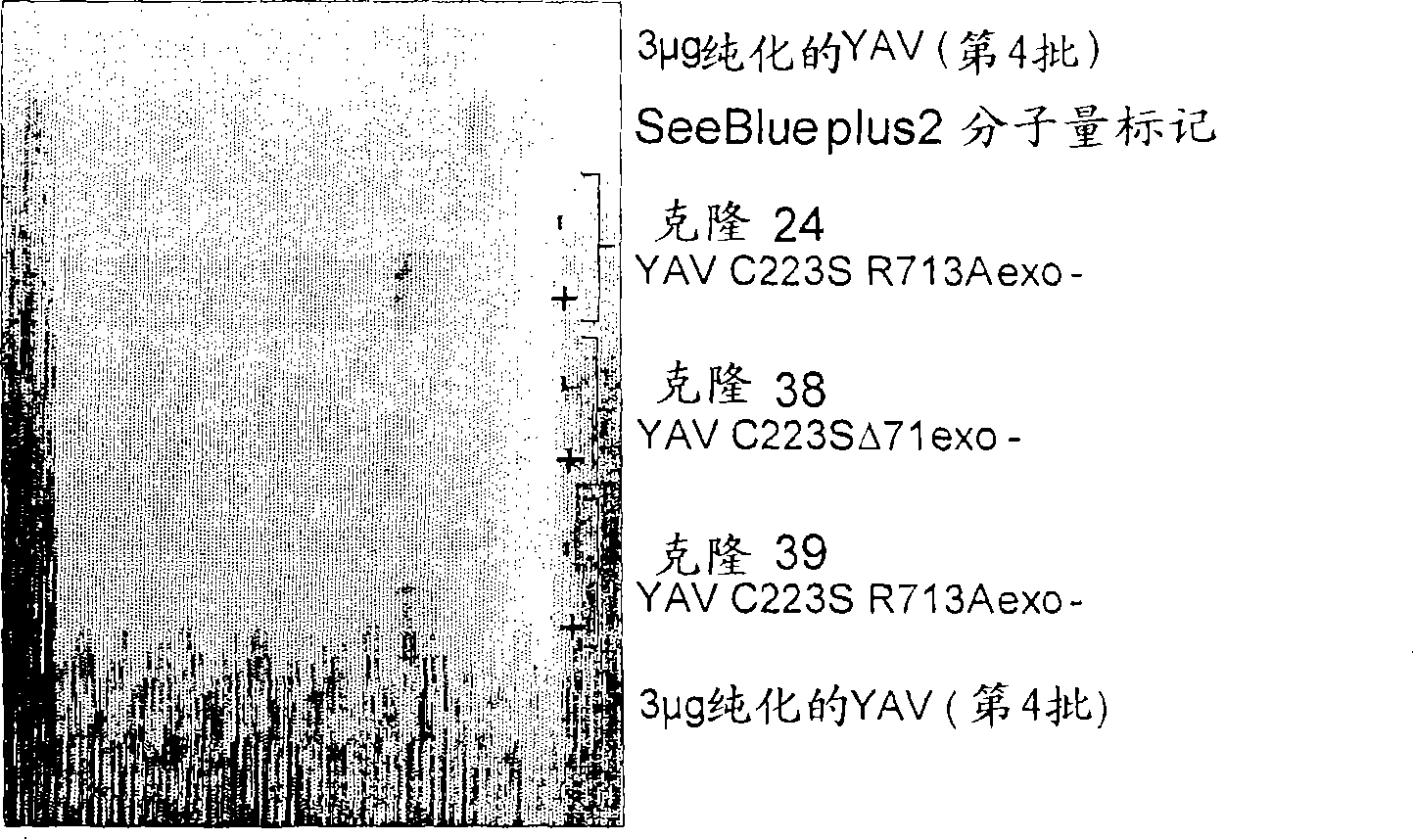
[0195] Example 1 - Preparation of altered polymerases
[0196] principle
[0197] A site-directed mutation was introduced in the C-terminal region of the 9°N-7YAV C223S polymerase, thereby reducing the affinity of the enzyme to DNA (wild-type 9°N-7 polymerase has a very high DNA affinity, Kd=50pM; Southworth et al. 1996 .PNAS.93, 5281).
[0198]An energy-minimized overlap alignment using the crystal structure of the open form of the 9°N-7 DNA polymerase (PDB=1qht), the open structure of the closely related DNA polymerase RB69 (PDB=1ih7), and the closed form of RB69 (PDB=1ig9) ( by Cresset) as a structural model to identify key residues involved in DNA binding. The crystal structure of the occluded form of RB69 polymerase (Franklin et al. 2001. Cell 105, 657) identified a number of residues that form hydrogen bonds or electrostatic interactions with complexed DNA, either directly with the nucleotide bases or with the phosphate backbone. A significant number of these resi...
Embodiment 2
[0222] Example 2 - NUNC tube assay using crude protein preparation.
[0223] A 5 ml small culture of the mutant enzyme (while using a YAV C223S exo-culture for direct comparison) was subjected to flash purification up to the heat treatment step as described in WO2005 / 024010. At this point, the sample was considered pure enough to be tested for activity.
[0224] Each crude preparation was buffer exchanged with enzymatic buffer (50 mM Tris pH 8.0, 6 mM MgSO4, 1 mM EDTA, 0.05% Tween20) using a S300 gel filtration spin column. This sample was not normalized to concentration. The assay used was simple incorporation of ffTTP into surface coupled A template hairpins. According to the product instructions, 2 pmol of 5'-amino oligomer 815
[0225] (5'-CGATCACGATCACGATCACGATCACGATCACGATCACGATCACGCTGATGTGCATGCTGTTGTTTTTTTACAACAGCATGCACATCAGCG-3') (SEQ ID NO: 12)
[0226] Coupled with NUNC-nucleolink gel strips.
[0227] After washing, each well was incubated with 20 μl aliquots o...
Embodiment 3
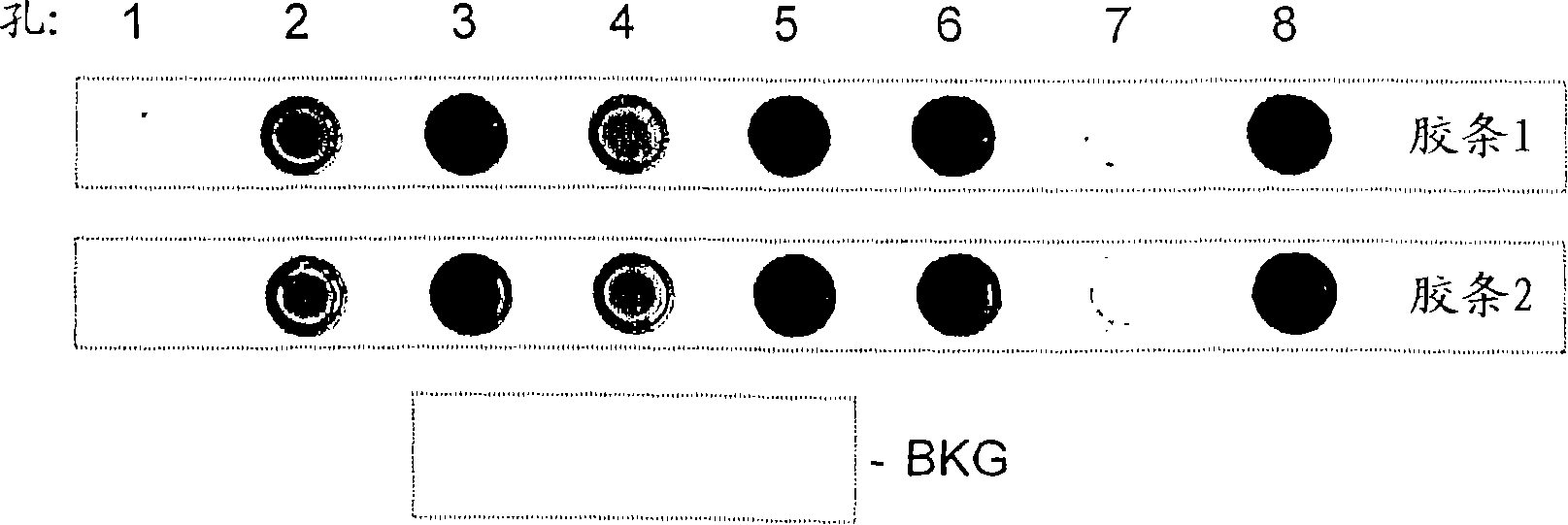
[0241] Example 3 - Single base incorporation assay
[0242] The activity of crude enzyme preparations (normalized concentrations) was measured using the single base incorporation assay described in WO2005 / 024010. In 2μM ffT-N3-cy3 and 20nM 10A hairpin DNA ( 32 P-labeled) were incubated with 30 or 3 μg / ml crude enzyme preparation for 10 min, and aliquots of the reaction mixture were removed at 0, 30, 60, 180 and 600 sec and run on a 12% acrylamide gel. Electrophoresis.
[0243] result
[0244] The gel image is shown in Figure 3.
[0245] Use Imagequant to quantify the band intensity, and the fluorescence intensity plotted against the incubation time is shown in Figure 4 time course.
[0246] These data give an estimate of the ffTTP first base incorporation performance of the mutant enzymes relative to YAV. Due to normalization to concentration, the activities are directly comparable. The Δ71 mutant was essentially inactive (kobs 21% of that observed with YAV), R743A a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com