Double-shell medicine sustained and controlled releasing carrier material and preparation method and application thereof
A technology of controlled release carrier and double shell layer, which is applied to the preparation of double shell drug sustained and controlled release carrier material, and the field of double shell drug sustained and controlled release carrier material, which can solve the problem of poor ability to control drug release and limit drug loading. and other problems, to achieve the effect of selective release, strong biocompatibility and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] step 1)
[0034] Preparation cephradine aqueous solution, concentration is 15mg / ml. 0.2g of hollow silica microsphere dry powder (average particle diameter is 200nm, average shell thickness is 20nm, the shell layer has mesoporous property, and average pore diameter is 10nm) is ultrasonically dispersed in the cephradine aqueous solution, stirred at room temperature for several days, and centrifuged to obtain The drug-loaded microspheres were centrifuged and washed 3 times to remove the drug molecules adsorbed on the surface. Oven-dried to obtain drug-loaded hollow silicon dioxide microsphere dry powder.
[0035] Step 2)
[0036] Prepare polyelectrolyte solutions. Chitosan was dissolved in acetic acid solution with a mass concentration of 1% to form solution A, and the concentration of chitosan was 10 mg / ml. Polyacrylic acid was dissolved in deionized water to form solution B, and the concentration of acrylic acid was 10mg / ml.
[0037] Disperse the drug-loaded hollow...
Embodiment 2
[0042] With 0.05g average diameter is the hollow silica microsphere of 350nm (average shell thickness is 25nm, shell layer has mesoporous property, and average aperture is 13.5nm) replaces the hollow silica microsphere in embodiment 1 step 1), With 10mg / ml polyallylamine hydrochloride (poly(allylamine hydrochloride), PAH) aqueous solution replaces solution A in embodiment 1 step 2), with 10mg / ml poly 4-styrene sulfonate (poly( styrenesulfonate), the aqueous solution of PSS) replaces the solution B in the step 2) of embodiment 1, and stirs at room temperature for 30 minutes to replace the 10 minutes in the step 2 of the embodiment 1), and replaces the pH in the step 2) of the embodiment 1 with deionized water= Acetic acid / sodium acetate standard buffer solution of 3.6 and acetic acid / sodium acetate standard buffer solution of pH=3.8. Other steps 1-3 of Example 1 were repeated. Finally, four double-layer polyelectrolyte multilayer membrane-coated composite cephradine-loaded mic...
Embodiment 3
[0045] The hollow silica microspheres (average shell thickness is 15nm, and the shell has mesoporous properties, and the average pore diameter is 7nm) with an average particle diameter of 100nm replace the hollow silica microspheres in step 1) of Example 1, with 10mg The aqueous solution of polydiallyldimethylammonium chloride (poly(diallyldimethylammonium chloride), PDDA) replaces the solution A in step 2) of embodiment 1, and replaces the step 2 of embodiment 1 with 10mg / ml sodium alginate aqueous solution ), stirred at room temperature for 60 minutes instead of 10 minutes in step 2) of embodiment 1, and replaced the aqueous solution of cephradine in step 1) of embodiment 1 with 2.5 mg / ml cisplatin saline solution. Other steps 1-3 of Example 1 were repeated. Finally, four composite cisplatin-loaded microspheres coated with double-layer polyelectrolyte multilayer films were obtained.
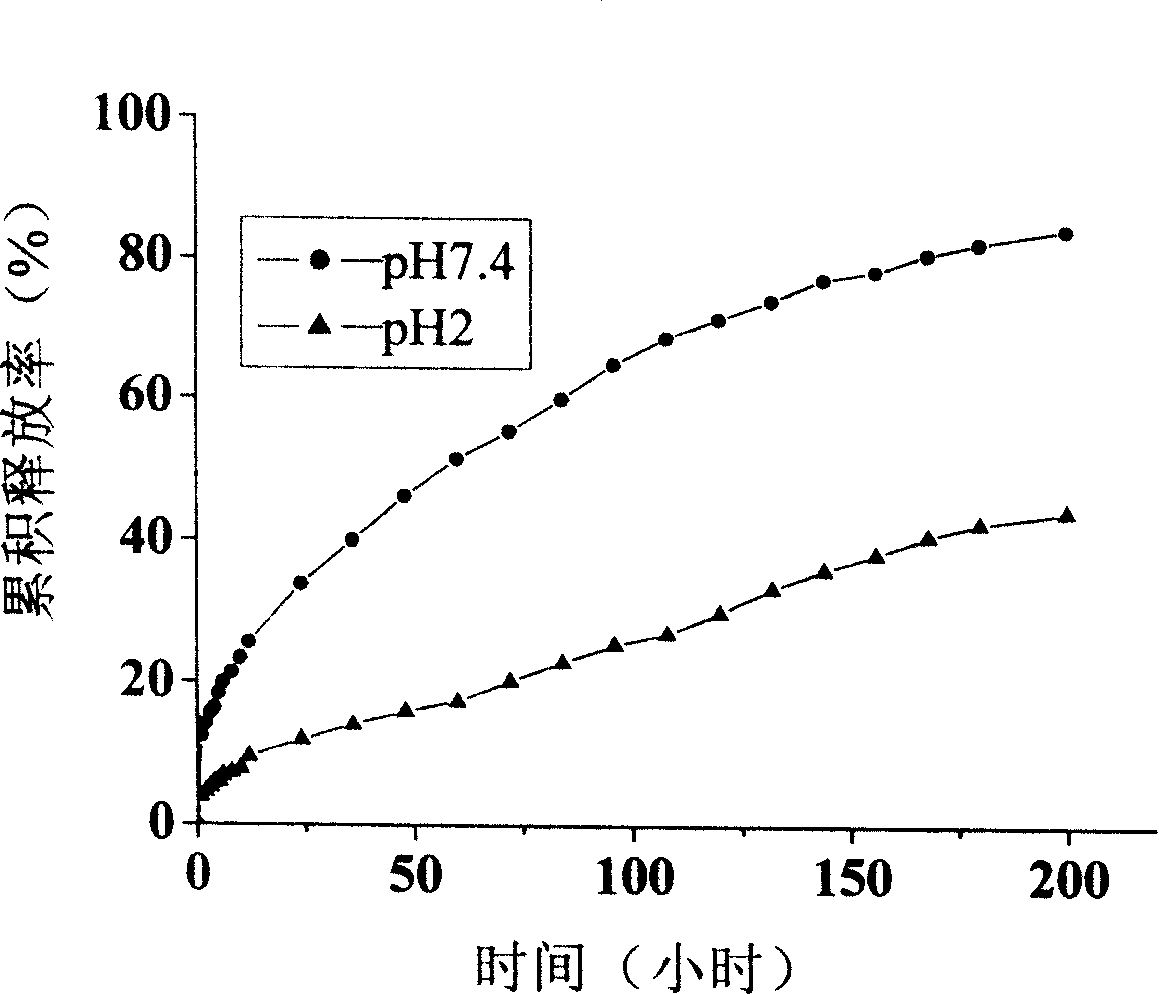
[0046] The drug release performance evaluation method is the same as in Example 1. The res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com