Composition containing antidementia agent
A composition and technology for dementia, applied in the field of compositions containing anti-dementia drugs, can solve the problems of failure to improve compliance, difficulty in controlling two or more anti-dementia drugs at the same time, and achieve excellent compliance and quality, simple Convenient production method, release easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
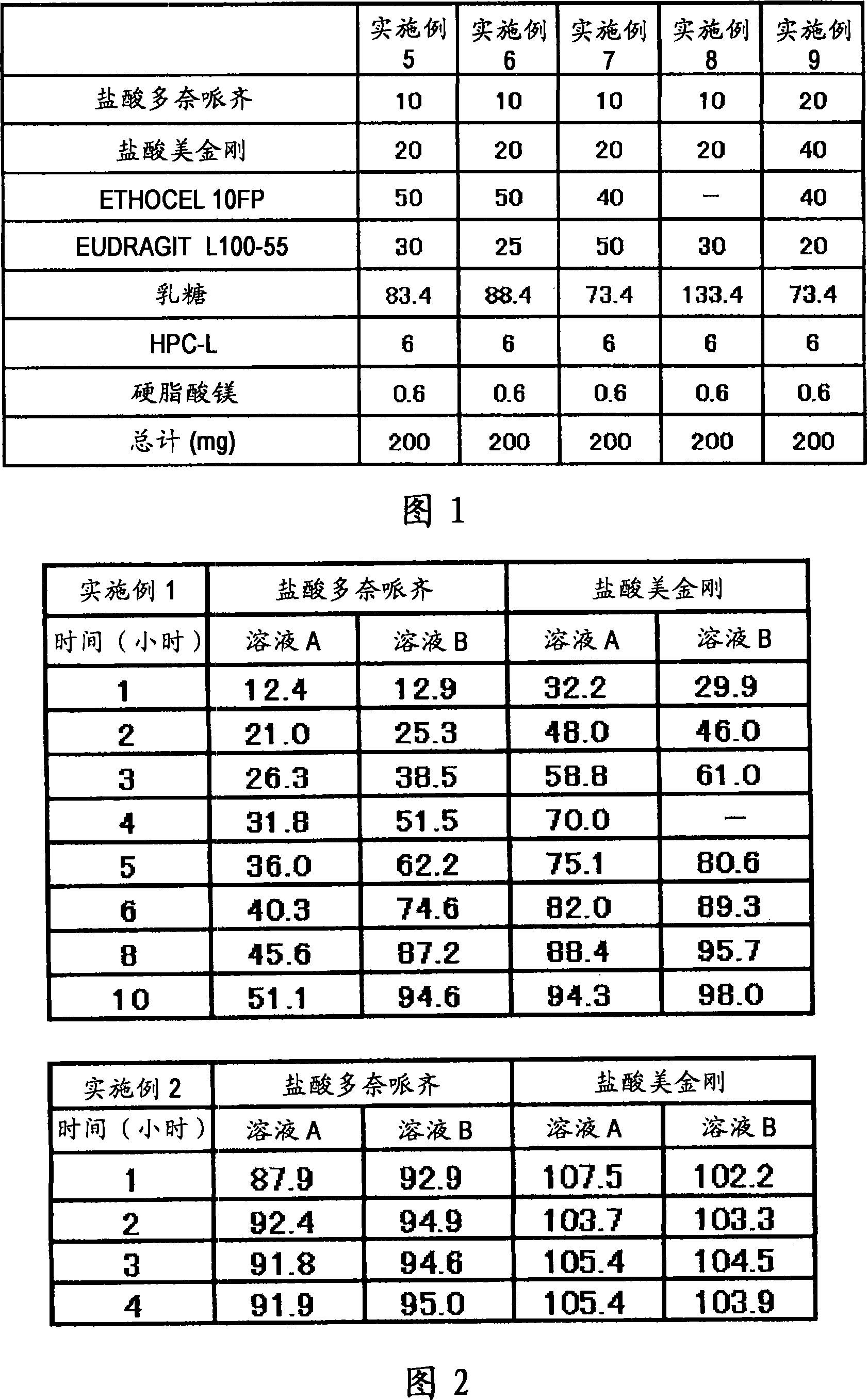
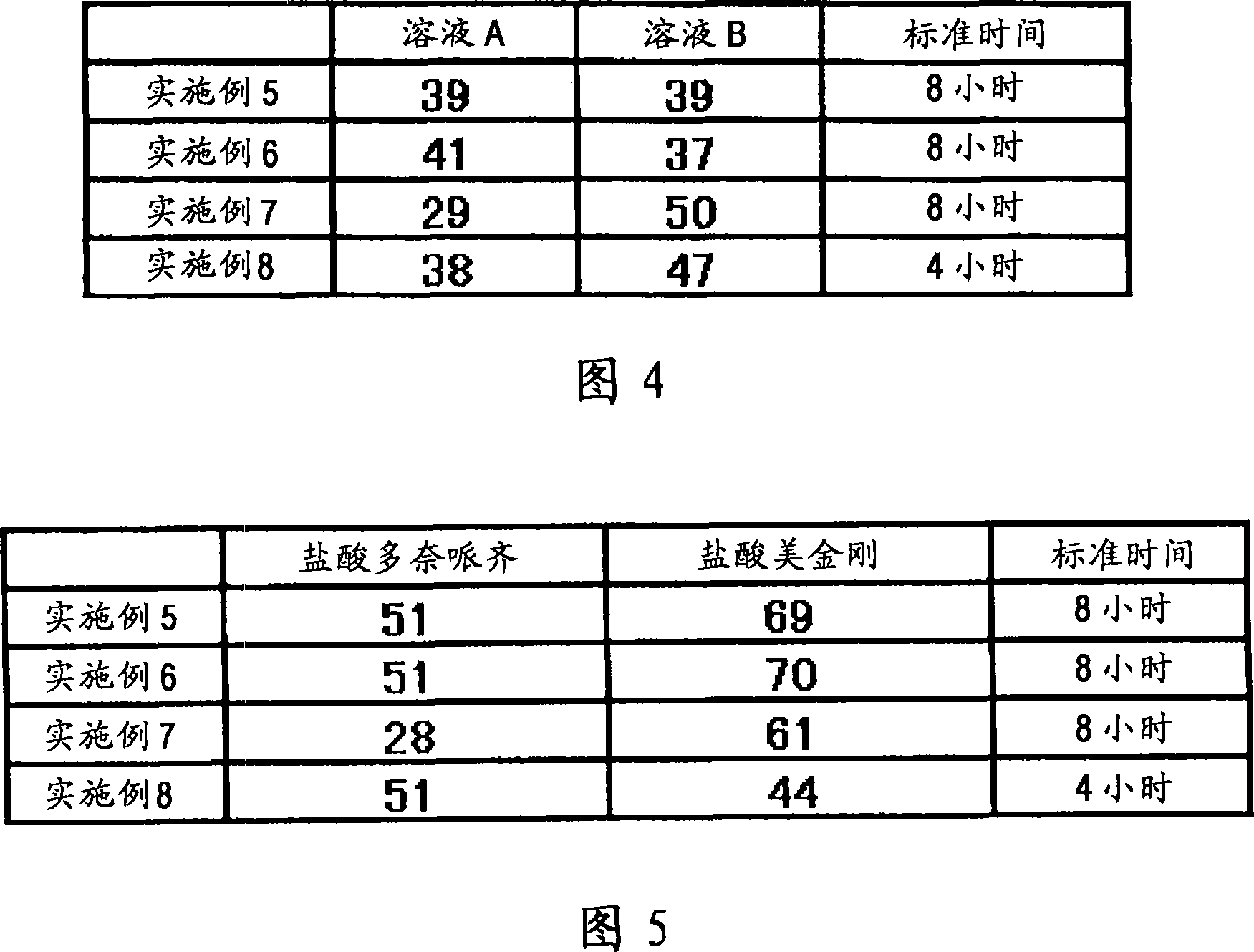
[0060] A fifth example is a composition containing multiple types of particles. Each type of granule can be manufactured as immediate release, sustained release, pulsed release or similar to freely establish the desired dissolution profile. For example, (1) the composition may contain immediate-release granules containing memantine hydrochloride, sustained-release granules containing donepezil hydrochloride and pulse-release granules containing memantine hydrochloride, so that after one administration, the blood concentration of memantine hydrochloride reaches The time interval between peaks can reach 8 hours or more, and donepezil hydrochloride is gradually released after administration. Alternatively, (2) a preparation form for once-a-day administration may be prepared, the preparation method comprising: making the sustained-release granules which start to be released 2 hours, 4 hours, 6 hours or 8 hours after administration contain 5 mg Adamantine, which is combined with g...
Embodiment 1
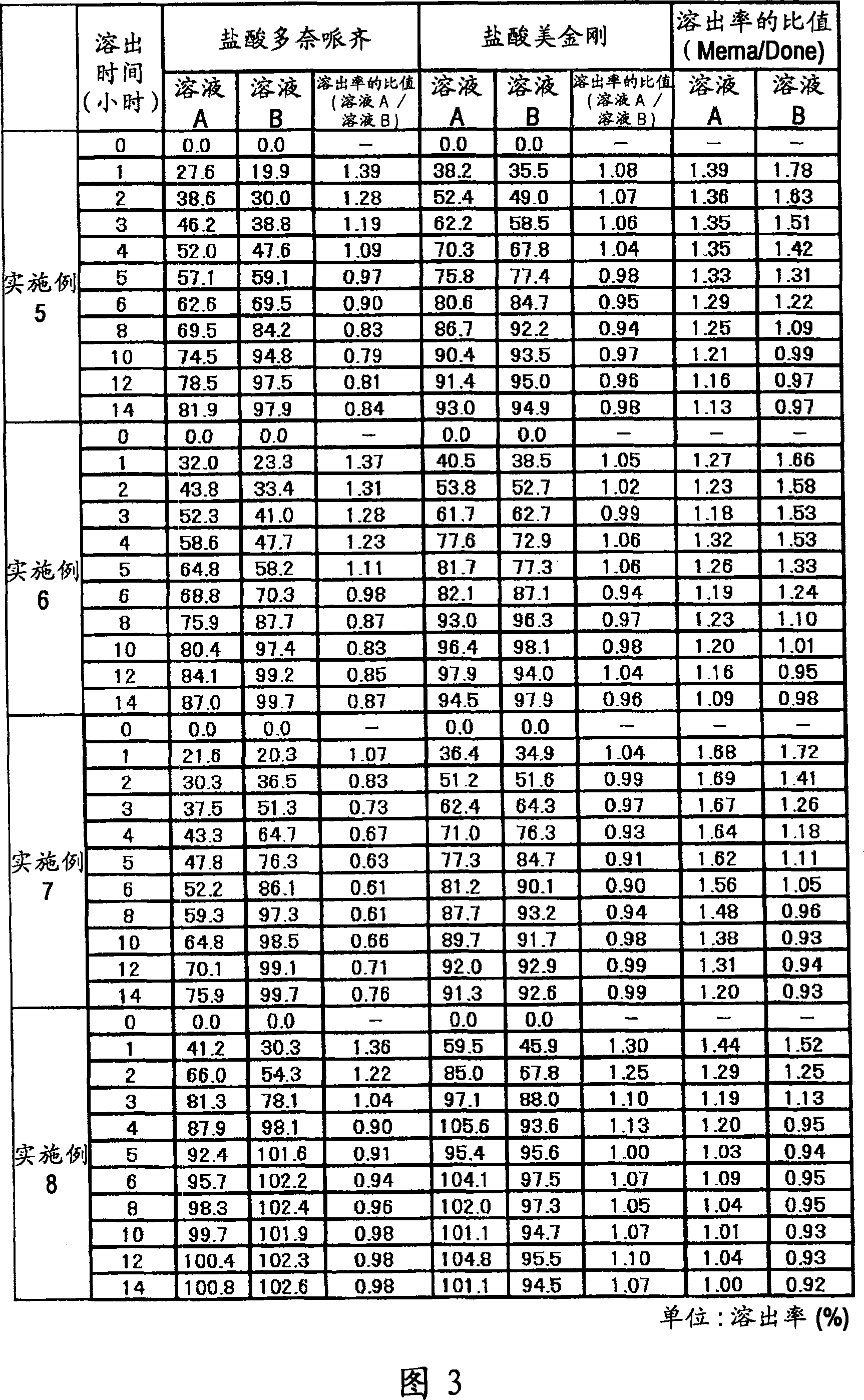
[0130] 6 g of donepezil hydrochloride (Eisai Co. Ltd.), 12 g of memantine hydrochloride (Lachema s.r.o.), 28.8 g of Ethocel 10FP (ethylcellulose, Dow Chemical Company), 36 g of Eudragit L100-55 (Röhm GmbH & Co.KG) and 45.6 lactose are mixed with each other in a granulator. An aqueous solution of 2.4 g of hydroxypropyl cellulose dissolved in an appropriate amount of pure water was added to the mixture for wet granulation, and the obtained granules were heated and dried by a disc dryer, and then sieved to obtain granules of the desired size. After sieving, 1 g of magnesium stearate was added per 109 g of granules and mixed, followed by tableting using a rotary tablet machine, thereby obtaining a compression-molded product with a diameter of 8 mm, which contained 10 mg of donepezil hydrochloride and 20 mg of memantine hydrochloride in a 220 mg tablet . Adopt Opadry yellow (Colorcon Japan Limited) to provide water-soluble film coating to the obtained product, the main component o...
Embodiment 2
[0132] 5 g of donepezil hydrochloride (Eisai Co. Ltd.), 10 g of memantine hydrochloride (Lachema s.r.o.), 20 g of cornstarch (Nihon Shokuhin Kako Co., Ltd.), 15 g of microcrystalline cellulose (Asahi Kasei Corporation) and 81.75 g of lactose in Mixed with each other in a granulator. An aqueous solution of 3.0 g of hydroxypropyl cellulose dissolved in an appropriate amount of pure water was added to the mixture for wet granulation, and the obtained granules were heated and dried by a disc dryer, and then sieved to obtain granules of the desired size. After sieving, 0.25 g of magnesium stearate was added per 134.75 g of granules and mixed, followed by tableting using a rotary tablet machine, thereby obtaining a compression-molded product with a diameter of 7 mm, which contained 5 mg of donepezil hydrochloride and 10 mg of hydrochloric acid in a 135 mg tablet Memantine. Adopt Opadry yellow (Colorcon Japan Limited) to provide water-soluble film coating to the obtained product, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com