Method for detecting content of fenthion
A technology for fenthion content, which is applied in the field of detection of fenthion content, can solve the problems that EMIT sensitivity assessment has not yet revealed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
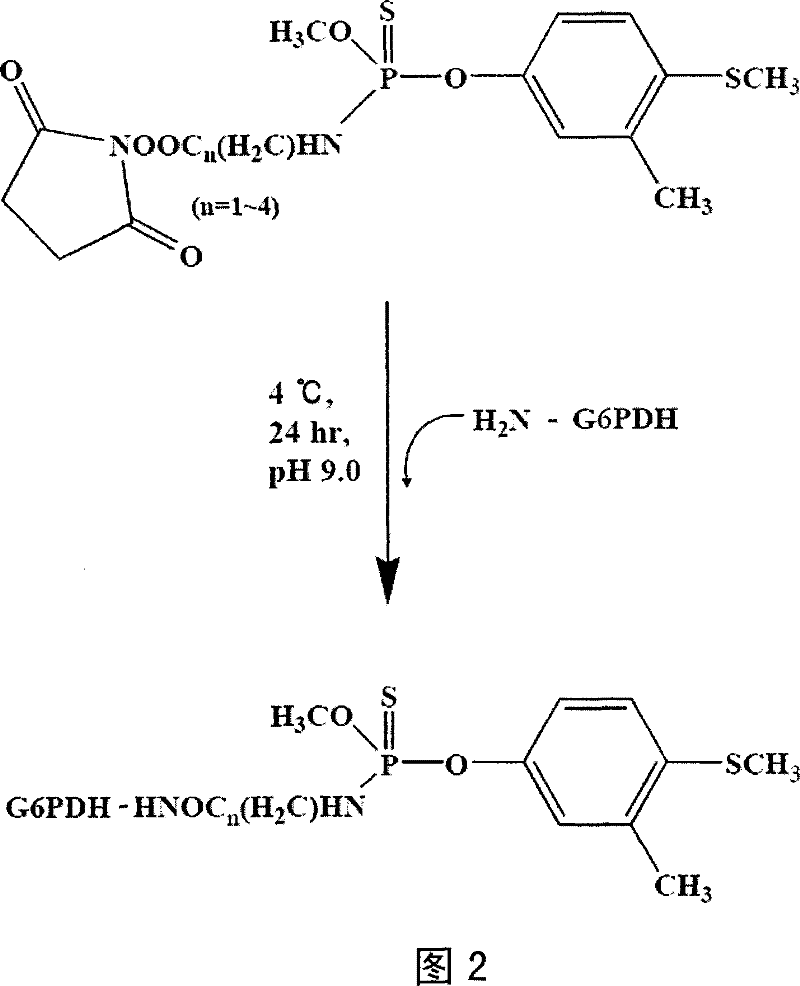
[0018] Example 1: Preparation of G6PDH-hapten conjugates
[0019] (1) Chemicals
[0020] Glucose-6-phosphate dehydrogenase (G6PDH), glucose-6-phosphate (G6P), β-nicotinamide adenine dinucleotide (NAD) from Leucinostoc mensenteroiders + ), oxaloacetic acid (OAA), and reduced NAD + Form (ie NADH), which was obtained from Sigma (St. Louis, MO), and fenthion (fenthion) was purchased from Dr. Ehrenstorfer (Augsburg, Germany). Coupling buffers for enzyme-hapten conjugates were 0.10M sodium bicarbonate buffer, pH 9.0. The working assay buffer for G6PDH-hapten conjugation is 0.05M tris(hydroxymethyl)aminomethane-hydrochloric acid buffer (tris(hydroxyl)aminomethane-hydrochloricacid buffer), pH 7.8 , containing 0.1M NaCl, 0.01% (w / v) NaN 3 , and 0.3% (w / v) gelatin (gelatin). All buffers were prepared using deionized water, each experiment was repeated three times and the median values are shown.
[0021] (2) Synthesis of hapten and production of antibodies
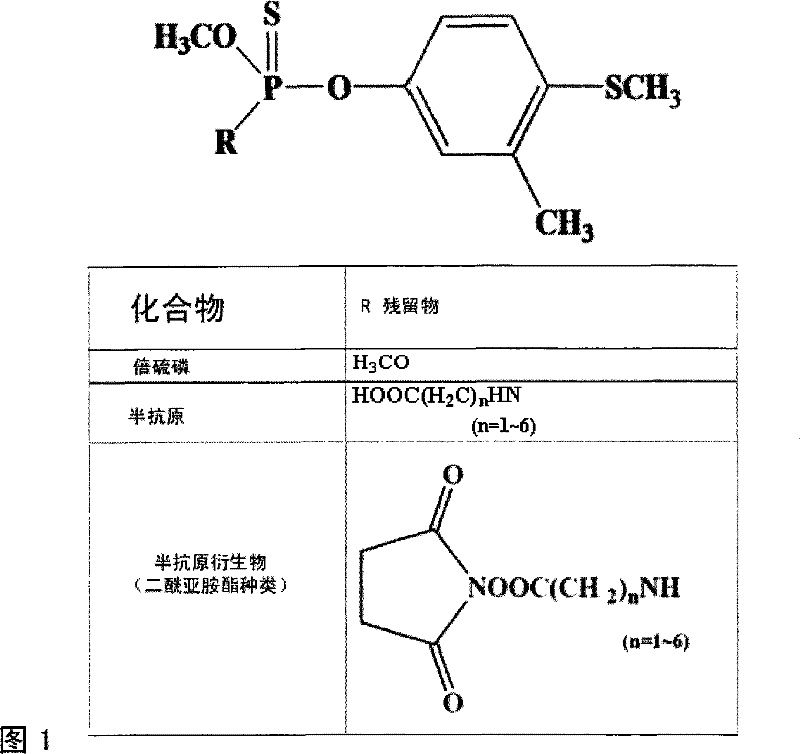
[0022] see figure ...
Embodiment 2
[0033] Example 2: Determination of enzyme activity and maximum percent inhibition of G6PDH-hapten conjugates.
[0034] (1) Instrument
[0035] Enzyme activity was determined by a Gilford-Stasaar-III spectrophotometer (spectrophotometer), which was equipped with a vacuum-operated sampling system (vacuum-operated sampling system) and temperature-controlled cuvette (temperature-controlled cuvette) (during the whole experiment maintained at 30C). This spectrophotometer is connected to Syva CP-5000 EMIT ClinicalProcessor (Mountain View, CA), a kind of clinical processor, is used for setting the record of interval reading (reading intervals) and absorbance value (absorbance values) automatically, utilizes Pierce company ( Rockford, IL) produced a microdialyzer system (microdialyzer system 500), used for dialyzer-hapten conjugates.
[0036] (2) Determination of enzyme activity
[0037] The activity of the G6PDH-hapten conjugate, which was detected by adding 100 μl of NAD + (32.5m...
Embodiment 3
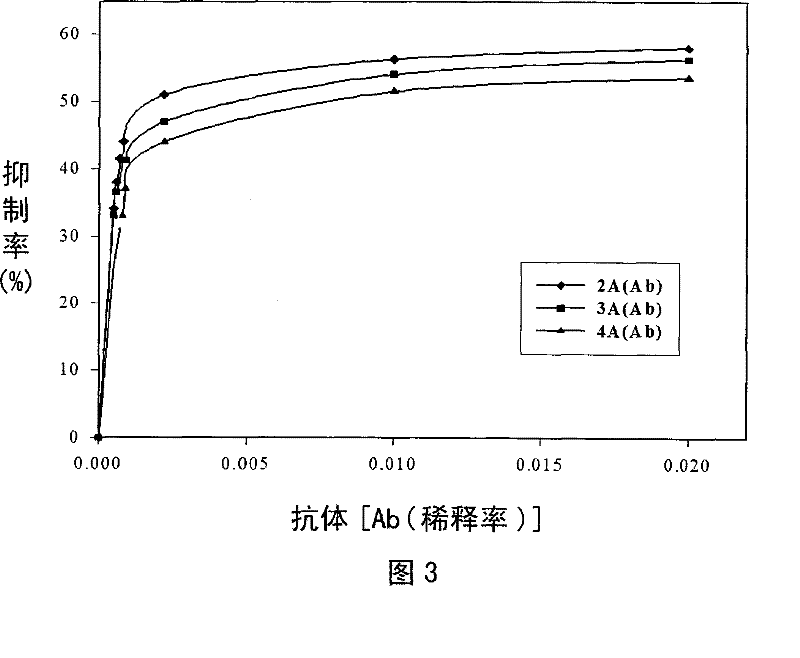
[0050] Example 3: Kinetic studies on inhibition by G6PDH-hapten conjugates.
[0051] (1) Combined kinetic research
[0052] The binding rate between the 6PDH-hapten conjugate and the anti-hapten antibody was determined using different incubation times (incubation time), and the detection solution contained 100 μl of the appropriately diluted conjugate (1 / 100 dilutions; 8.96×10 -7 concentration of M), and 100 μl of antibody solution (diluted 1 / 1250) were mixed and incubated for 0 to 30 minutes. After each incubation period, the enzymatic activity of the conjugates was determined by addition of substrate, as described above, and kinetic curves were developed by plotting the rate of inhibition versus the position of the incubation period.
[0053] (2) The influence of the concentration of different antibodies and G6PDH-hapten conjugates for inhibition.
[0054] Using an Aliquots type aliquot injector (100 μl), the antibody solution containing different antibodies with differe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com